4.3 Tertiary Structure: Water-Soluble Proteins Fold into Compact Structures
As already discussed, primary structure is the sequence of amino acids, and secondary structure is the simple repeating structures formed by hydrogen bonds between hydrogen and oxygen atoms of the peptide backbone. Another level of structure, tertiary structure, refers to the spatial arrangement of amino acid residues that are far apart in the sequence and to the pattern of disulfide bonds. This level of structure is the result of interactions between the R groups of the peptide chain. To explore the principles of tertiary structure, we will examine myoglobin, the first protein to be seen in atomic detail.
Myoglobin Illustrates the Principles of Tertiary Structure
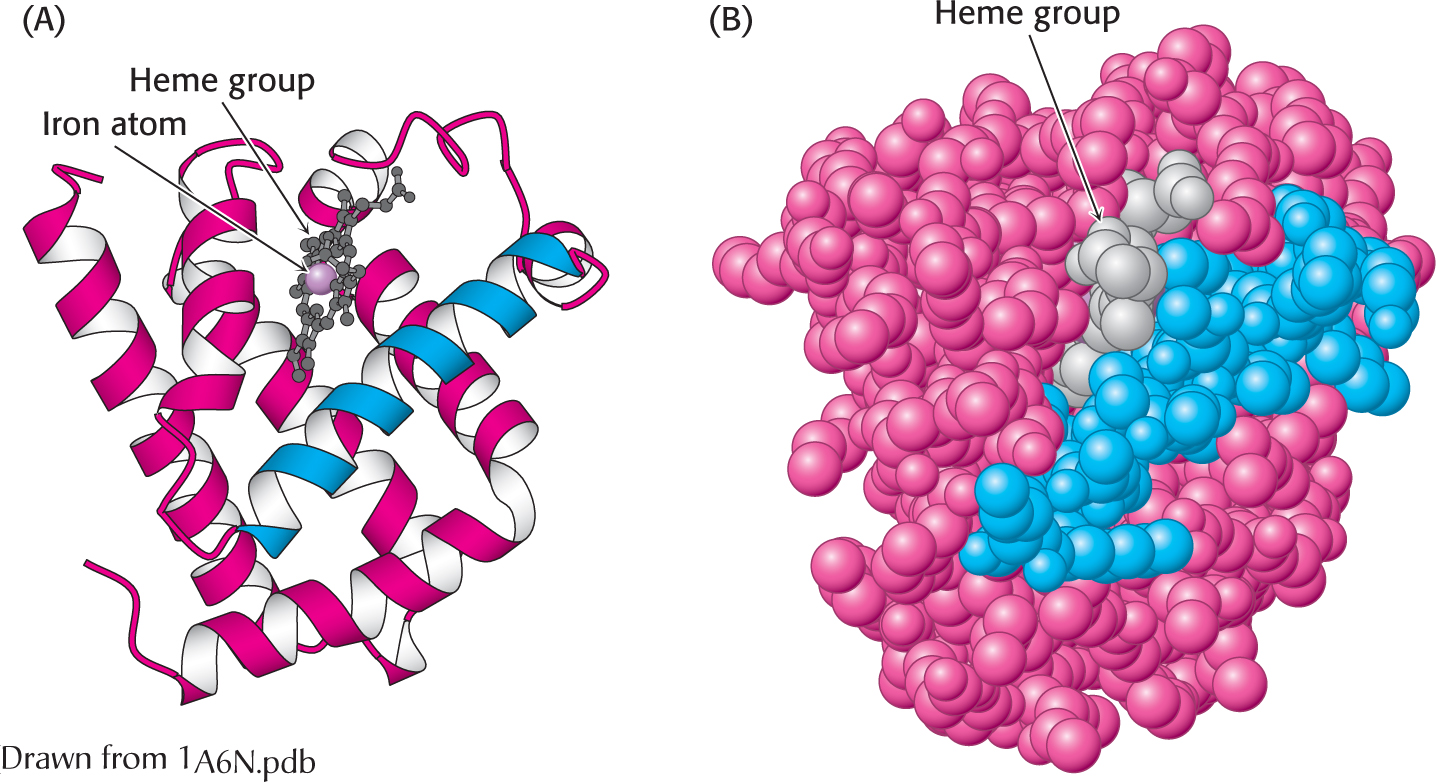
Myoglobin is an example of a globular protein (Figure 4.25). In contrast with fibrous proteins such as keratin, globular proteins have a compact three-

 The three-
The three-Myoglobin, a single polypeptide chain of 153 amino acids, is an oxygen-
Myoglobin, like most other proteins, is asymmetric because of the complex folding of its main chain. A unifying principle emerges from the distribution of side chains. The striking fact is that the interior consists almost entirely of nonpolar residues (Figure 4.26). The only polar residues on the interior are two histidine residues, which play critical roles in binding the heme iron and oxygen. The outside of myoglobin, on the other hand, consists of both nonpolar and polar residues, which can interact with water and thus render the molecule water soluble. The space-
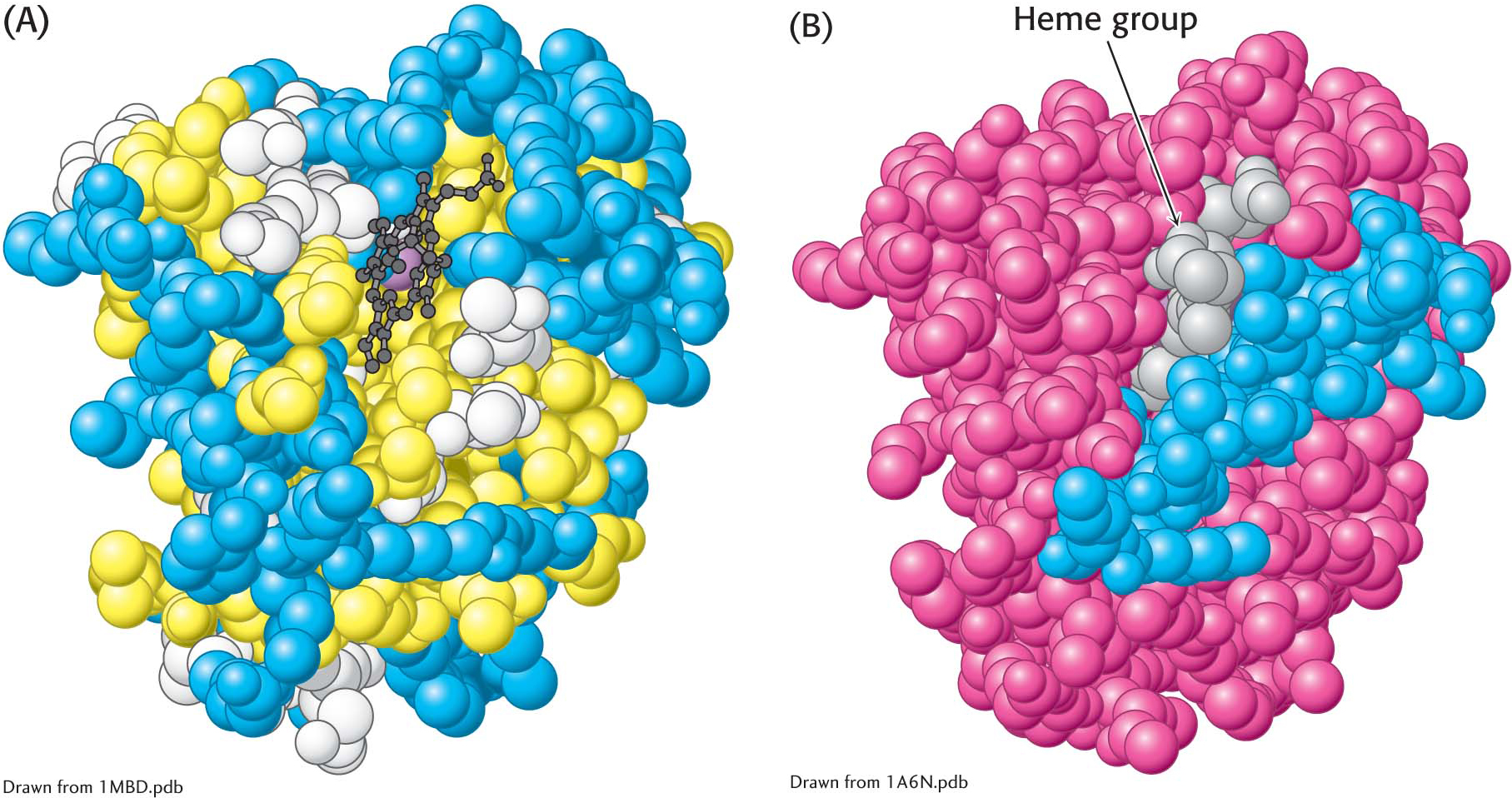

 The distribution of amino acids in myoglobin. (A) A space-
The distribution of amino acids in myoglobin. (A) A space-This contrasting distribution of polar and nonpolar residues reveals a key facet of protein architecture. In an aqueous environment such as the interior of a cell, protein folding is driven by the hydrophobic effect—
Some proteins that span biological membranes are “the exceptions that prove the rule” because they have the reverse distribution of hydrophobic and hydrophilic amino acids. For example, consider porins, proteins found in the outer membranes of many bacteria. Membranes are built largely of the hydrophobic hydrocarbon chains of lipids (Chapter 12). Thus, porins are covered on the outside largely by hydrophobic residues that interact with the hydrophobic environment. In contrast, the center of the protein contains many charged and polar amino acids that surround a water-
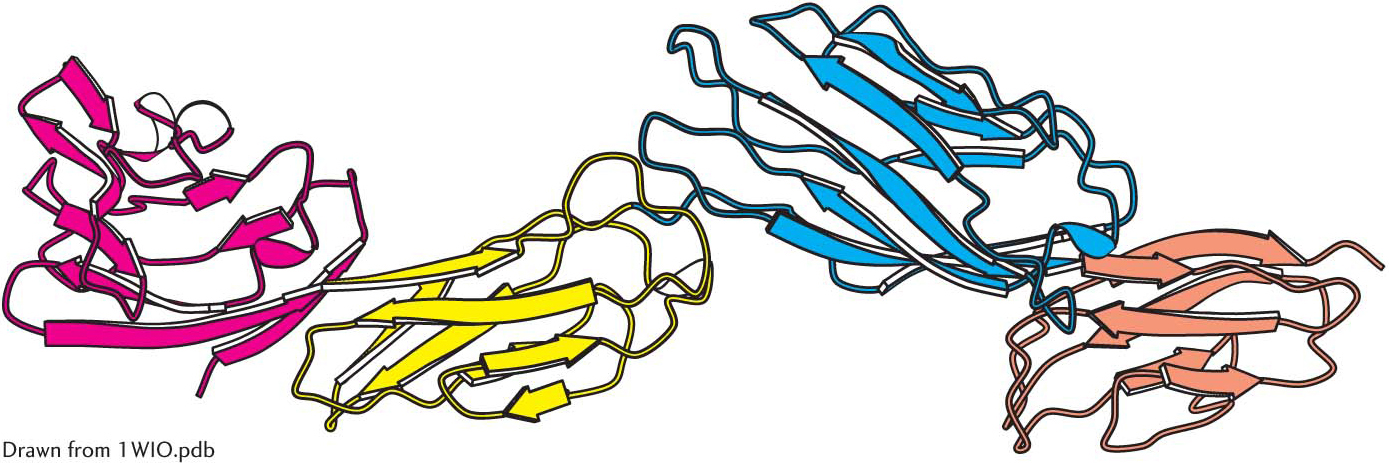
The Tertiary Structure of Many Proteins Can Be Divided into Structural and Functional Units
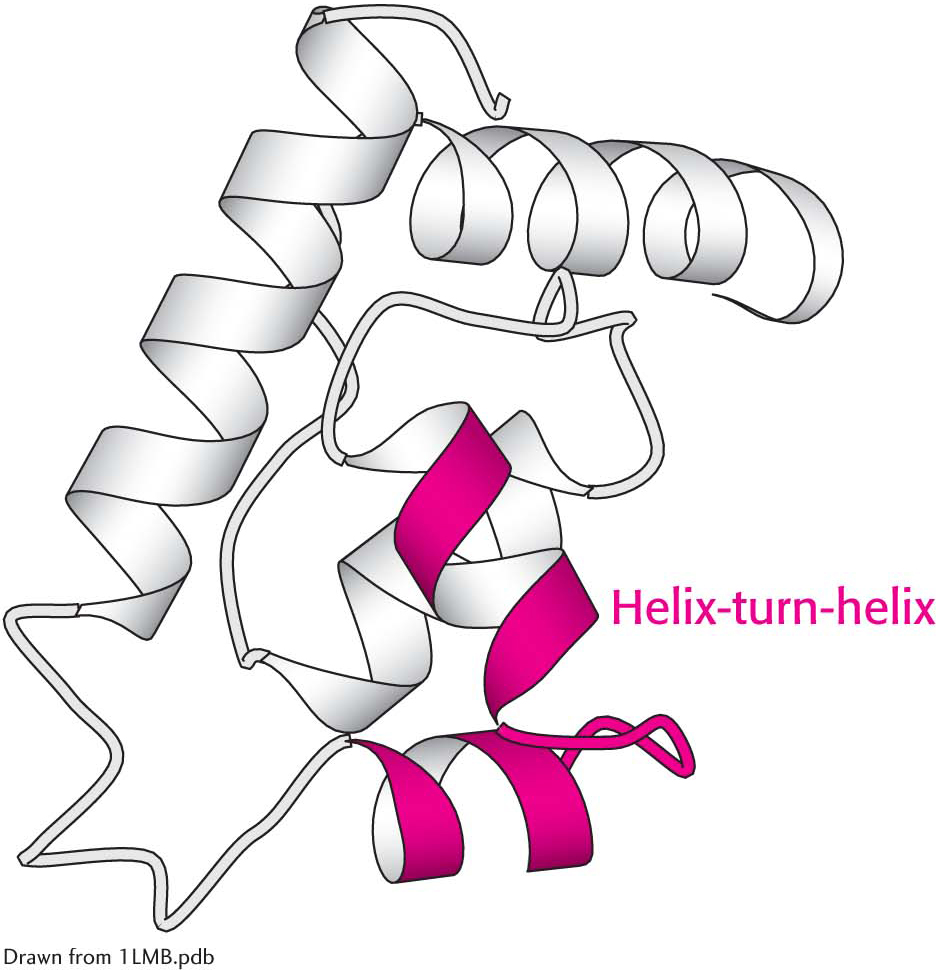
Certain combinations of secondary structure are present in many proteins and frequently exhibit similar functions. These combinations are called motifs or supersecondary structures. For example, an α helix separated from another α helix by a turn, called α helix-turn-
Some polypeptide chains fold into two or more compact regions that may be connected by a flexible segment of polypeptide chain, rather like pearls on a string. These compact globular units, called domains, range in size from about 30 to 400 amino acid residues. For example, the extracellular part of CD4, a cell-