
6.1 Enzymes Are Powerful and Highly Specific Catalysts
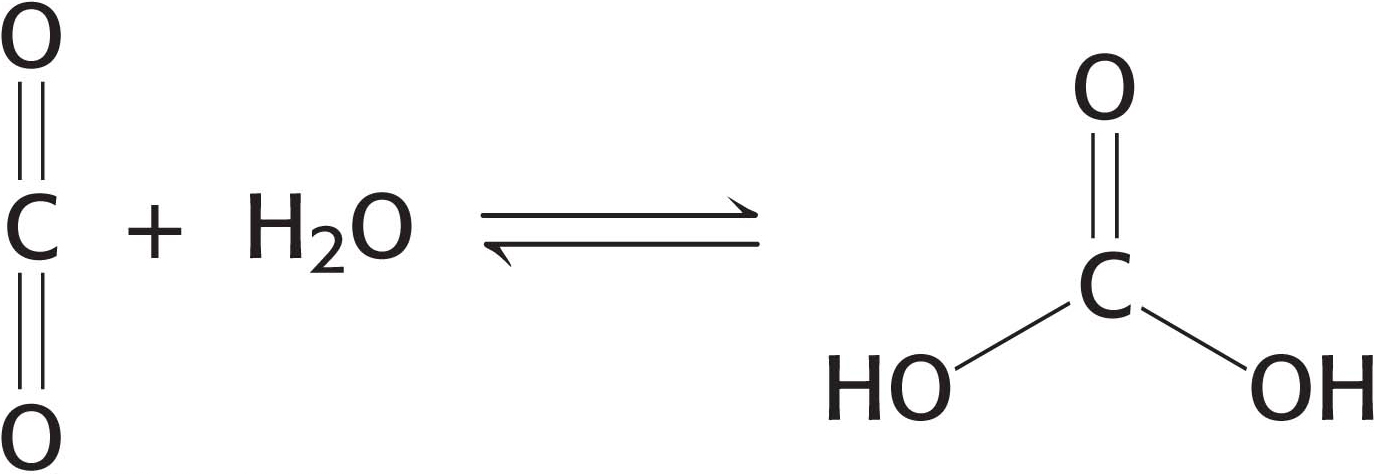
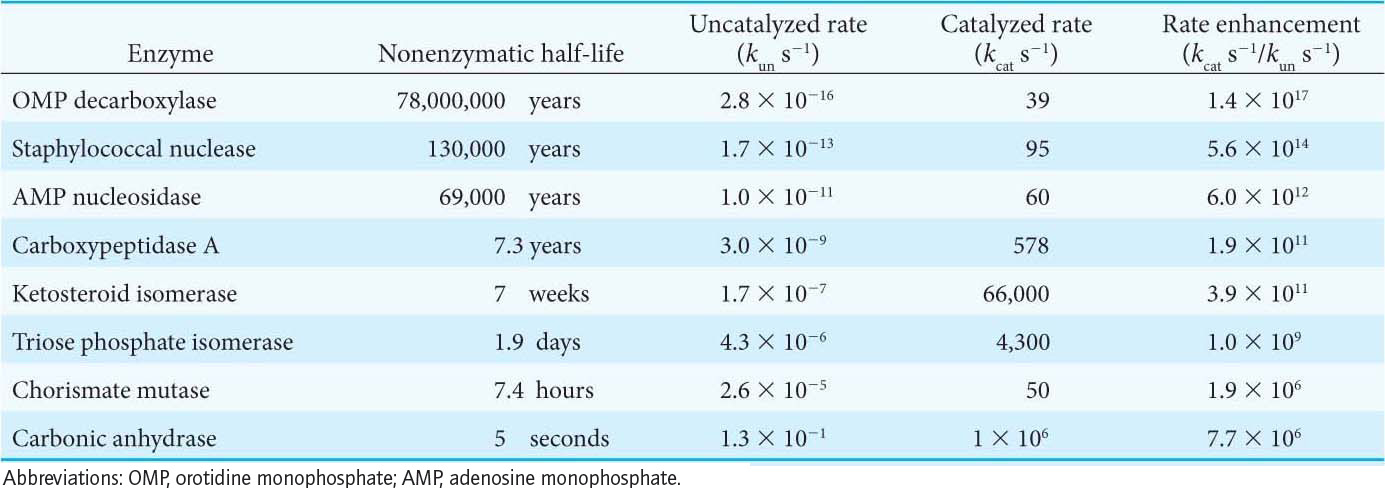
Enzymes accelerate the rate of reactions by factors of as much as a million or more (Table 6.1). Indeed, most reactions in biological systems do not take place at perceptible rates in the absence of enzymes. Even a reaction as simple as adding water to carbon dioxide is catalyzed by an enzyme—
Proteolytic Enzymes Illustrate the Range of Enzyme Specificity
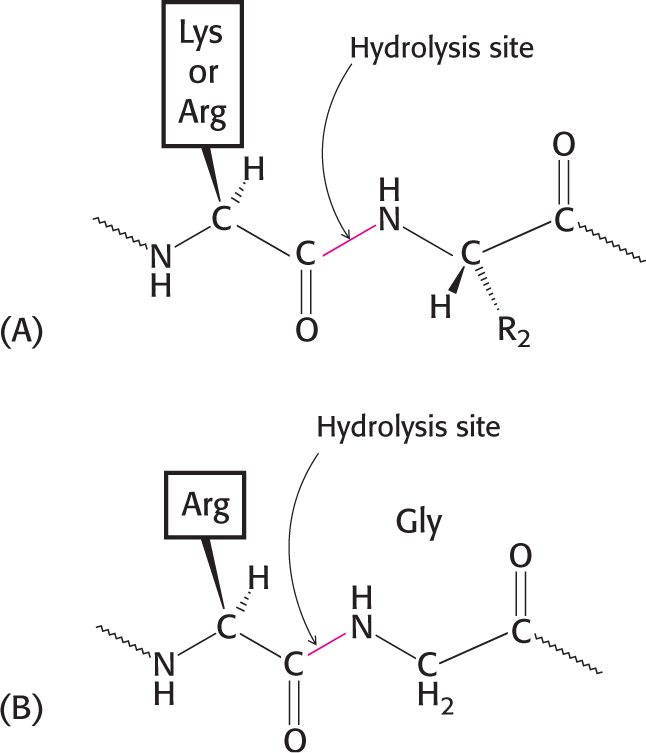
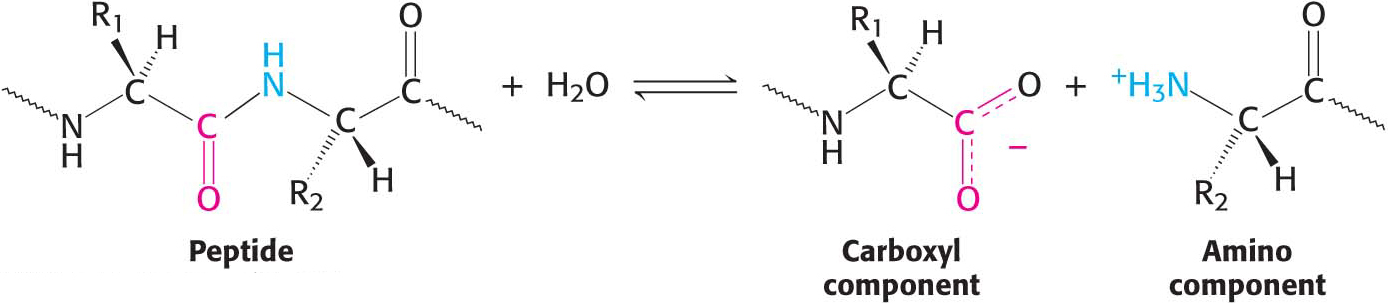
Enzymes are highly specific both in the reactions that they catalyze and in their choice of reactants, which are called substrates. An enzyme usually catalyzes a single chemical reaction or a set of closely related reactions. Let us consider proteolytic enzymes as an example. The biochemical function of these enzymes is to catalyze proteolysis, the hydrolysis of a peptide bond:
Proteolytic enzymes differ markedly in their degree of substrate specificity. Papain, which is found in papaya plants, is quite undiscriminating: it will cleave any peptide bond with little regard to the identity of the adjacent side chains. This lack of specificity accounts for its use in meat-
There Are Six Major Classes of Enzymes
More than a thousand enzymes have been identified—
Oxidoreductases. These enzymes transfer electrons between molecules. In other words, these enzymes catalyze oxidation–
reduction reactions. We will first meet a member of this class, lactate dehydrogenase, when we consider glycolysis, the first pathway in glucose degradation. Page 99Transferases. These enzymes transfer functional groups between molecules. Aminotransferases are prominent in amino acid synthesis and degradation, where they shuffle amine groups between donor and acceptor molecules.
Hydrolyases. A hydrolyase cleaves molecules by the addition of water. Trypsin, the proteolytic enzyme already discussed, is a hydrolyase.
Lyases. A lyase adds atoms or functional groups to a double bond or removes them to form double bonds. The lyase fumarase is crucial to aerobic fuel metabolism.
Isomerases. These enzymes move functional groups within a molecule. We will meet triose phosphate isomerase in glycolysis.
Ligases. Ligases join two molecules in a reaction powered by ATP hydrolysis. DNA ligase, an important enzyme in DNA replication, is representative of this class.
The classification of all enzymes into these six classes also allowed the development of a standard nomenclature for enzymes. Many enzymes have common names that provide little information about the reactions that they catalyze. Trypsin exemplifies this lack of information. Most other enzymes are named for their substrates and for the reactions that they catalyze, with the suffix “ase” added. Thus, a peptide hydrolase is an enzyme that hydrolyzes peptide bonds, whereas ATP synthase is an enzyme that synthesizes ATP. Common names will be used in this book, but let’s examine the more accurate nomenclature.
The six groups (classes) of enzymes were subdivided and further subdivided so that a four-
DID YOU KNOW?
Hydrolysis reactions, the breaking of a chemical bond by the addition of a water molecule, are prominent in biochemistry.
Consider trypsin as an example. Trypsin cleaves bonds by the addition of water; consequently, it is a member of group 3: Hydrolyases. Trypsin cleaves only peptide bonds, and hydrolyases that cleave peptide bonds are classified as 3.4. Trypsin employs a serine residue to facilitate hydrolysis and cleaves the protein chain internally (in contrast with the removal of amino acids from the end of the polypeptide chain). Such enzymes are placed in sub-