
6.4 Enzymes Facilitate the Formation of the Transition State
✓ 2 Explain the relation between the transition state and the active site of an enzyme, and list the characteristics of active sites.
The free-
A chemical reaction of substrate S to form product P goes through a transition state X‡ that has a higher free energy than does either S or P. The double dagger denotes the transition state. The transition state is a fleeting molecular structure that is no longer the substrate but is not yet the product. The transition state is the least-
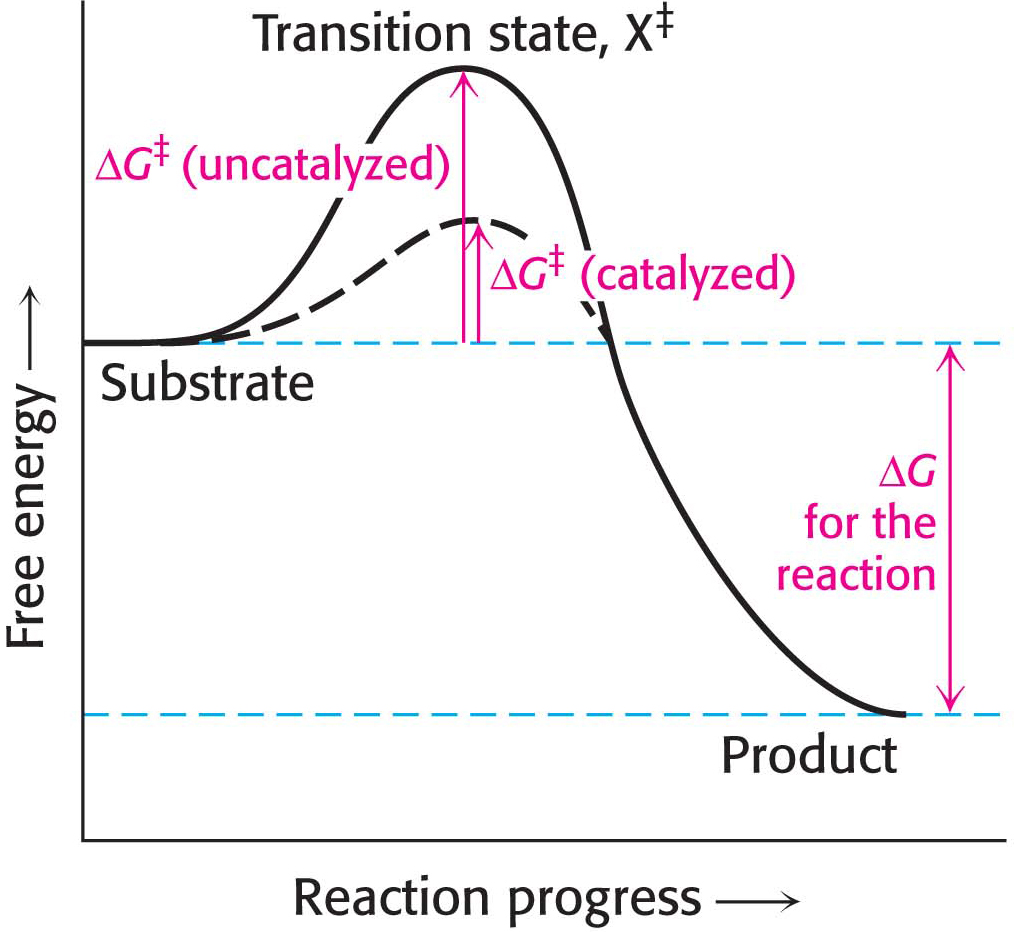
The difference in free energy between the transition state and the substrate is called the free energy of activation or simply the activation energy, symbolized by ΔG‡ (Figure 6.3):
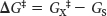
Note that the energy of activation, or ΔG‡, does not enter into the final ΔG calculation for the reaction, because the energy that had to be added to reach the transition state is released when the transition state becomes the product. The activation energy immediately suggests how enzymes accelerate the reaction rate without altering ΔG of the reaction: enzymes function to lower the activation energy. In other words, enzymes facilitate the formation of the transition state.
The combination of substrate and enzyme creates a reaction pathway whose transition-
The Formation of an Enzyme–Substrate Complex Is the First Step in Enzymatic Catalysis
Much of the catalytic power of enzymes comes from their binding to and then altering the structure of the substrate to promote the formation of the transition state. Thus, the first step in catalysis is the formation of an enzyme–
The Active Sites of Enzymes Have Some Common Features
The active site of an enzyme is the region that binds the substrates (and the cofactor, if any). It also contains the amino acid residues that directly participate in the making and breaking of bonds. These residues are called the catalytic groups. In essence, the interaction of the enzyme and substrate at the active site promotes the formation of the transition state. The active site is the region of the enzyme that most directly lowers the ΔG‡ of the reaction, thus providing the rate-
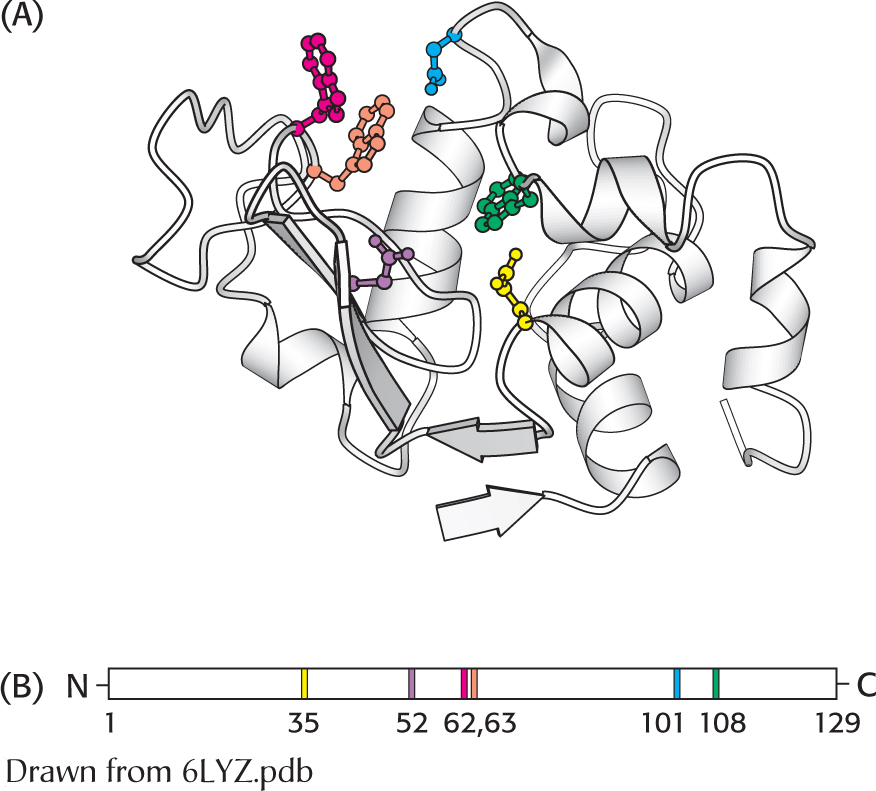 Figure 6.4
Figure 6.4 Active sites may include distant residues. (A) Ribbon diagram of the enzyme lysozyme with several components of the active site shown in color. (B) A schematic representation of the primary structure of lysozyme shows that the active site is composed of residues that come from different parts of the polypeptide chain.
Active sites may include distant residues. (A) Ribbon diagram of the enzyme lysozyme with several components of the active site shown in color. (B) A schematic representation of the primary structure of lysozyme shows that the active site is composed of residues that come from different parts of the polypeptide chain.
The active site is a three-
dimensional cleft or crevice formed by groups that come from different parts of the amino acid sequence: indeed, amino acids near to one another in the primary structure are often sterically constrained from adopting the structural relations necessary to form the active site. In lysozyme, the important groups in the active site are contributed by residues numbered 35, 52, 62, 63, 101, and 108 in the sequence of 129 amino acids (Figure 6.4). Lysozyme, found in a variety of organisms and tissues including human tears, degrades the cell walls of some bacteria.The active site takes up a small part of the total volume of an enzyme. Although most of the amino acid residues in an enzyme are not in contact with the substrate, the cooperative motions of the enzyme as a whole help to correctly position the catalytic residues at the active site. Experimental attempts to reduce the size of a catalytically active enzyme show that the minimum size requires about 100 amino acid residues. In fact, nearly all enzymes are made up of more than 100 amino acid residues, which gives them a mass greater than 10 kDa and a diameter of more than 25 Å, suggesting that all amino acids in the protein, not just those at the active site, are ultimately required to form a functional enzyme.
Active sites are unique microenvironments. The close association between the active site and the substrate means that water is usually excluded from the active site unless it is a reactant. The nonpolar microenvironment of the cleft enhances the binding of substrates as well as catalysis. Nevertheless, the cleft may also contain polar residues, some of which may acquire special properties essential for substrate binding or catalysis. The internal positions of these polar residues are biologically crucial exceptions to the general rule that polar residues are located on the surface of proteins, exposed to water.
Substrates are bound to enzymes by multiple weak attractions. The noncovalent interactions between the enzyme and the substrate in ES complexes are much weaker than covalent bonds. These weak reversible interactions are mediated by electrostatic interactions, hydrogen bonds, and van der Waals forces, powered by the hydrophobic effect. Van der Waals forces become significant in binding only when numerous substrate atoms simultaneously come close to many enzyme atoms. Hence, to bind as strongly as possible, the enzyme and substrate should have complementary shapes.
Page 105The specificity of binding depends on the precisely defined arrangement of atoms in an active site. Because the enzyme and the substrate interact by means of short-
range forces that require close contact, a substrate must have a matching shape to fit into the site. Emil Fischer’s analogy of the lock and key (Figure 6.5), expressed in 1890, has proved to be highly stimulating and fruitful. However, we now know that enzymes are flexible and that the shapes of the active sites can be markedly modified by the binding of substrate, a process of dynamic recognition called induced fit (Figure 6.6). Moreover, the substrate may bind to only certain conformations of the enzyme, in what is called conformation selection. Thus, the mechanism of catalysis is dynamic, involving structural changes with multiple intermediates of both reactants and the enzyme.
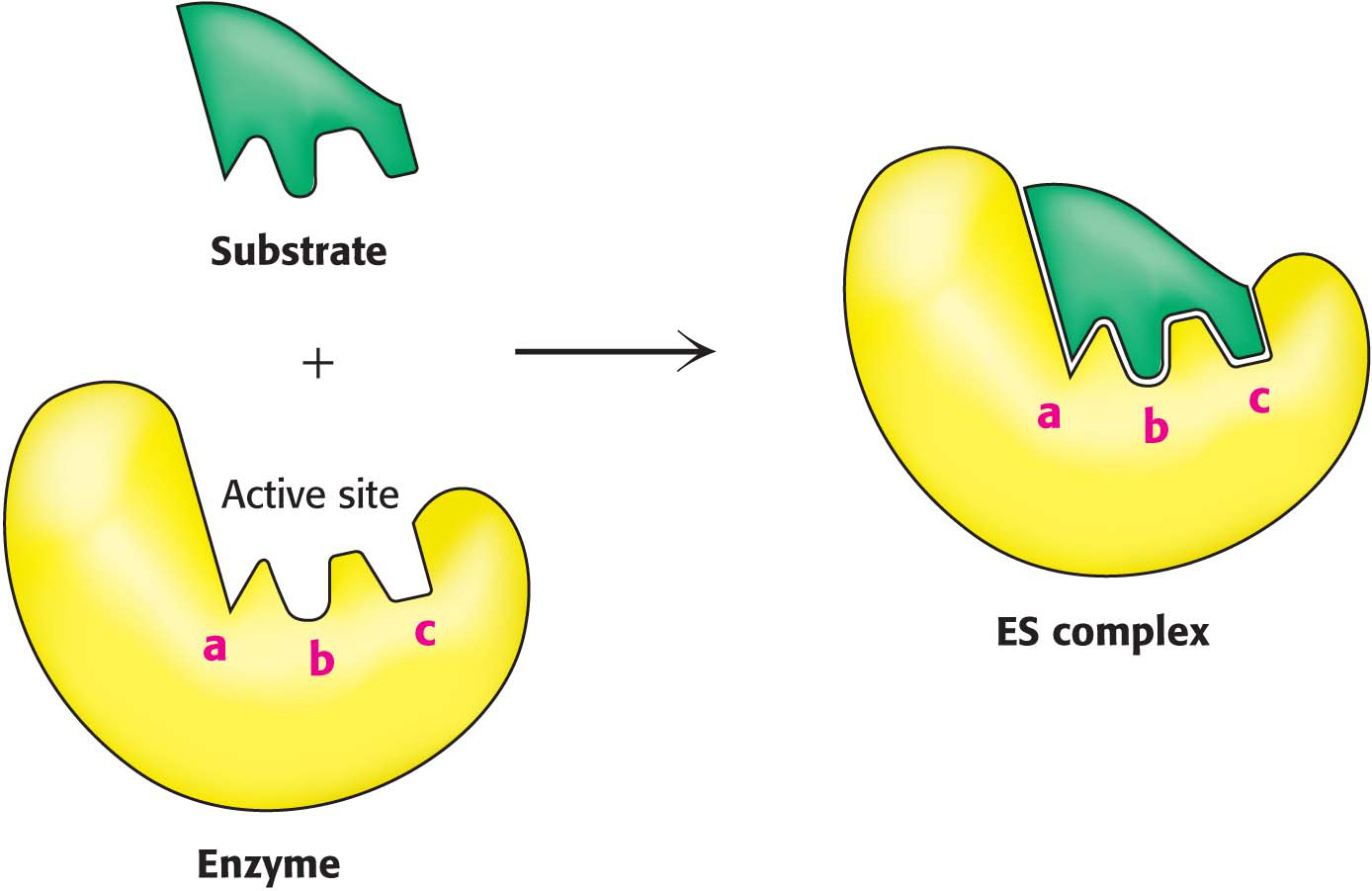
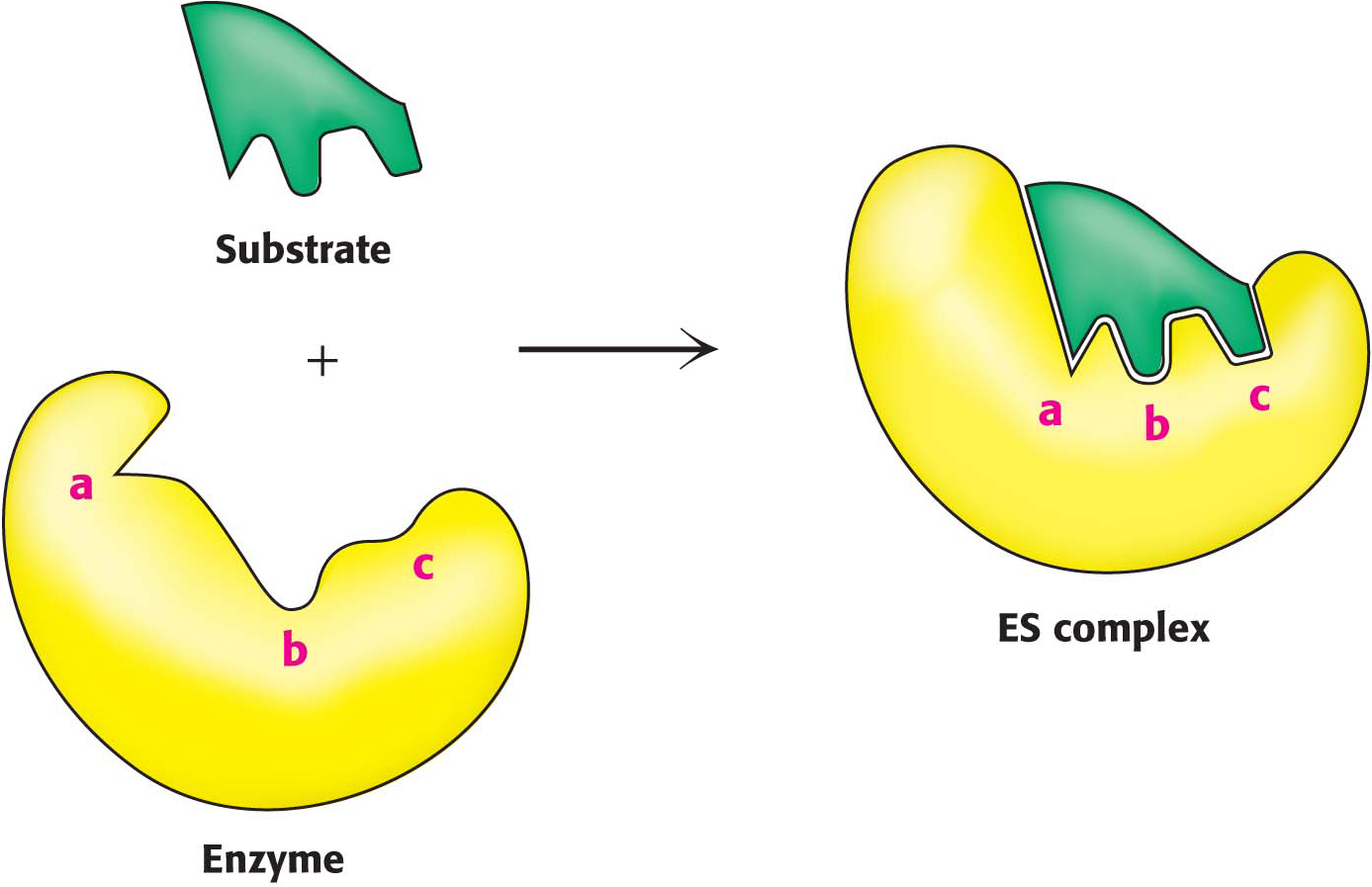
The Binding Energy Between Enzyme and Substrate Is Important for Catalysis
Enzymes lower the activation energy, but where does the energy to lower the activation energy come from? Free energy is released by the formation of a large number of weak interactions between a complementary enzyme and substrate. The free energy released on binding is called the binding energy. Only the correct substrate can participate in most or all of the interactions with the enzyme and thus maximize binding energy, accounting for the exquisite substrate specificity exhibited by many enzymes. Furthermore, the full complement of such interactions is formed only when the substrate is in the transition state. Thus, the maximal binding energy is released when the enzyme facilitates the formation of the transition state. The energy released by the interactions between the enzyme and the substrate can be thought of as lowering the activation energy. The interaction of the enzyme with the substrate and reaction intermediates is fleeting, with molecular movements resulting in the optimal alignment of functional groups at the active site so that maximum binding energy occurs only between the enzyme and the transition state, the least-
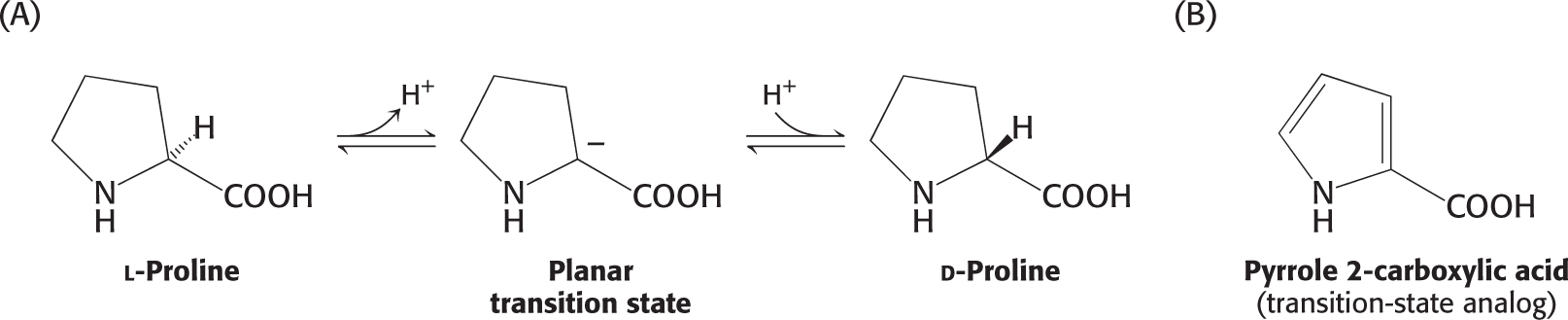
Transition-State Analogs Are Potent Inhibitors of Enzymes
The importance of the formation of the transition state to enzyme catalysis is demonstrated by the study of compounds that resemble the transition state of a reaction but are not capable of being acted on by the enzyme. These mimics are called transition-
DID YOU KNOW?
Racemization is the conversion of one enantiomer into another; in regard to proline, the interconversion of the L and D isomers.
If our understanding of the importance of the transition state to catalysis is correct, then antibodies that recognize transition states should function as catalysts. Antibodies have been generated that recognize the transition states of certain reactions, and these antibodies, called catalytic antibodies or abzymes, do indeed function as enzymes.