PROBLEMS
Question 6.1
1. Raisons d’être. What are the two properties of enzymes that make them especially useful catalysts? ✓ 1
Question 6.2
2. Shared properties. What are the general characteristics of enzyme active sites? ✓ 2
Question 6.3
3. Partners. What does an apoenzyme require to become a holoenzyme?
Question 6.4
4. Different partners. What are the two main types of cofactors?
Question 6.5
5. One a day. Why are vitamins necessary for good health?
Question 6.6
6. A function of state. What is the fundamental mechanism by which enzymes enhance the rate of chemical reactions? ✓ 1
Question 6.7
7. Nooks and crannies. What is the structural basis for enzyme specificity? ✓ 2
Question 6.8
8. Mutual attraction. What is meant by the term binding energy? ✓ 2
Question 6.9
9. Catalytically binding. What is the role of binding energy in enzyme catalysis? ✓ 2
Question 6.10
10. Sticky situation. What would be the result of an enzyme having a greater binding energy for the substrate than for the transition state? ✓ 2
Question 6.11
11. Made for each other. Match the term with the proper description.
Enzyme Substrate Cofactor Apoenzyme Holoenzyme Coenzymes ΔG°′ Transition state Active site Induced fit | The least- Site on the enzyme where catalysis takes place Function of 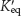 Enzyme plus cofactor Enzyme minus its cofactor Protein catalyst A coenzyme or metal Reactant in an enzyme- Small vitamin- Change in enzyme structure |
Question 6.12
12. Give with one hand, take with the other. Why does the activation energy of a reaction not appear in the final ΔG of the reaction? ✓ 1
Question 6.13
13. Making progress. The illustrations below show the reaction-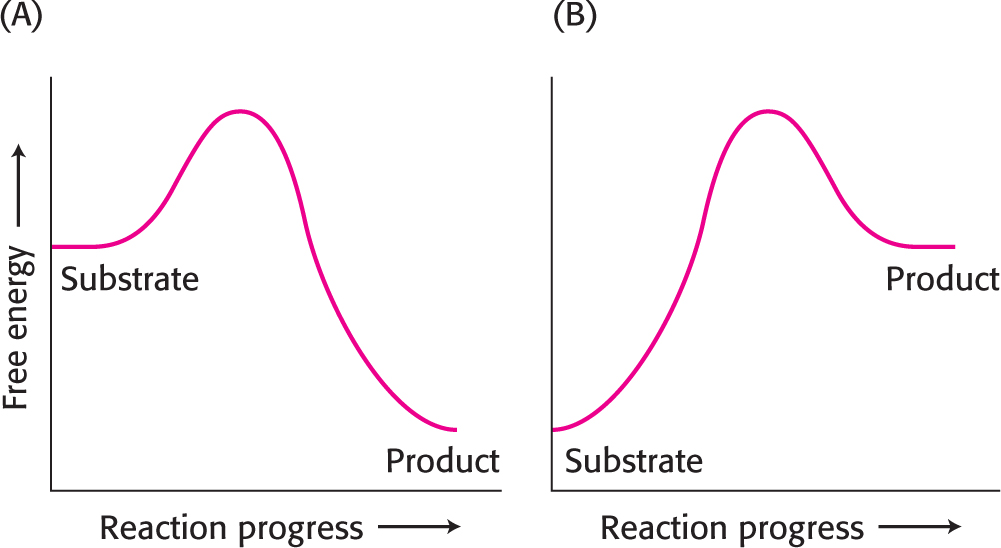
Question 6.14
14. Mountain climbing. Proteins are thermodynamically unstable. The ΔG of the hydrolysis of proteins is quite negative, yet proteins can be quite stable. Explain this apparent paradox. What does it tell you about protein synthesis?
Question 6.15
15. Protection. Suggest why the enzyme lysozyme, which degrades cell walls of some bacteria, is present in tears.
Question 6.16
16. Stability matters. Transition-
Question 6.17
17. Match’em. Match the K′eq values with the appropriate ΔG°′ (kJ mol−1) values. ✓ 1
1 10−5 104 102 10−1 | −11.42 5.69 −22.84 28.53 0 |
Question 6.18
18. Free energy! Consider the following reaction:
After the reactants and products were mixed and allowed to reach equilibrium at 25°C, the concentration of each compound was measured: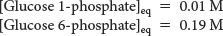
Calculate Keq and ΔG°′.
Question 6.19
19. More free energy! The isomerization of dihydroxyacetone phosphate (DHAP) to glyceroldehyde 3-
Question 6.20
20. A tenacious mutant. Suppose that a mutant enzyme binds a substrate 100 times as tightly as does the native enzyme. What is the effect of this mutation on catalytic rate if the binding of the transition state is unaffected? ✓ 2
Question 6.21
21. A question of stability. Pyridoxal phosphate (PLP) is a coenzyme for the enzyme ornithine aminotransferase. The enzyme was purified from cells grown in PLP-
(a) Why does the amount of active enzyme decrease with the time of incubation?
(b) Why does the amount of enzyme from the PLP-
Challenge Problems
Question 6.22
22. Free energy, yet again. Assume that you have a solution of 0.1 M glucose 6-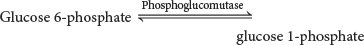
The ΔG°′ for the reaction is +7.5 kJ mol−1 (+1.8 kcal mol−1).
(a) Does the reaction proceed as written, and, if so, what are the final concentrations of glucose 6-
(b) Under what cellular conditions could you produce glucose 1-
Question 6.23
23. Potential donors and acceptors. The hormone progesterone contains two ketone groups. At pH 7, which side chains of a protein might form hydrogen bonds with progesterone?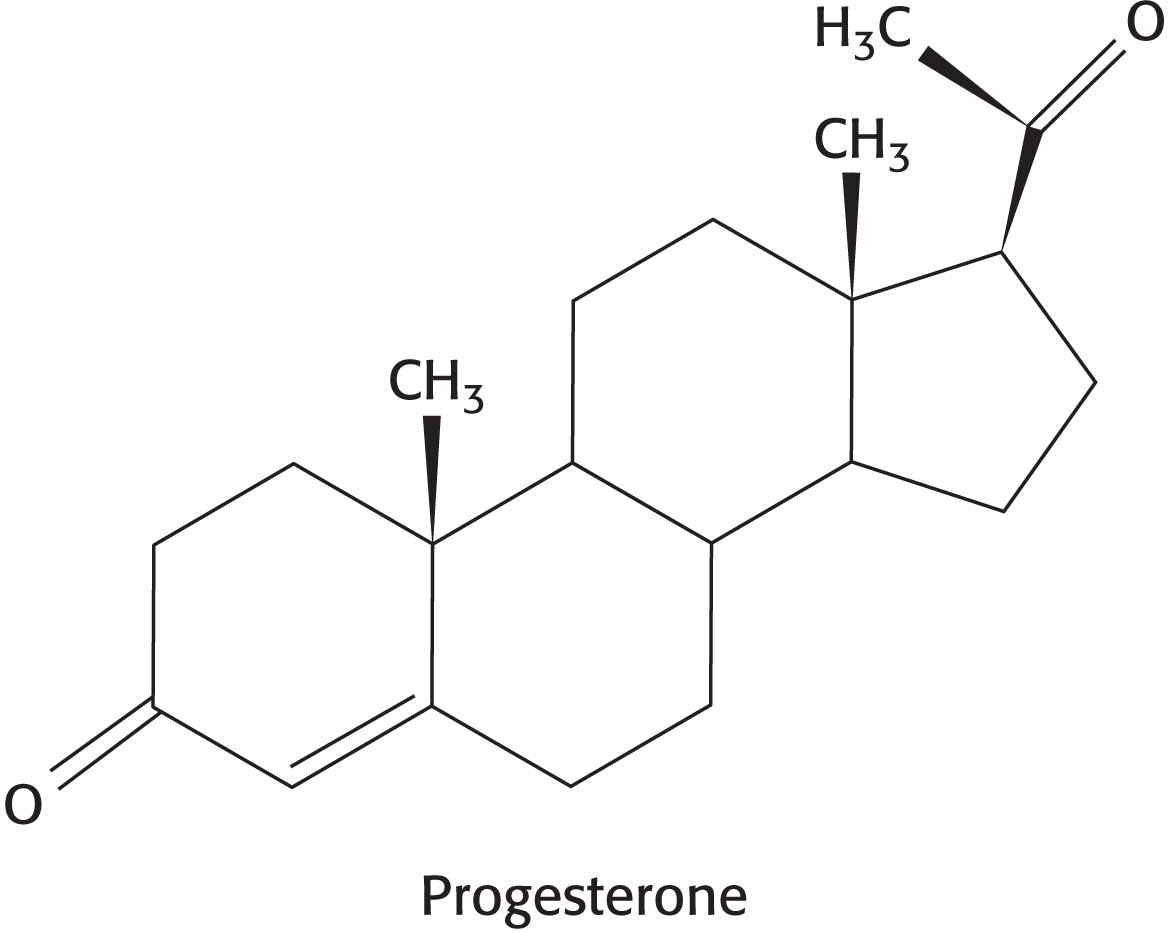
Question 6.24
24. The more things change, the more they stay the same. Suppose that, in the absence of enzyme, the forward rate constant (kF) for the conversion of S into P is 10−4 s−1 and the reverse rate constant (kR) for the conversion of P into S is 10−6 s−1:
(a) What is the equilibrium for the reaction? What is the ΔG°′?
(b) Suppose an enzyme enhances the rate of the reaction 100-
Selected Readings for this chapter can be found online at www.whfreeman.com/