
7.3 Allosteric Enzymes Are Catalysts and Information Sensors
✓ 5 Identify the key properties of allosteric proteins, and describe the structural basis for these properties.
Enzymes that conform to simple Michaelis–
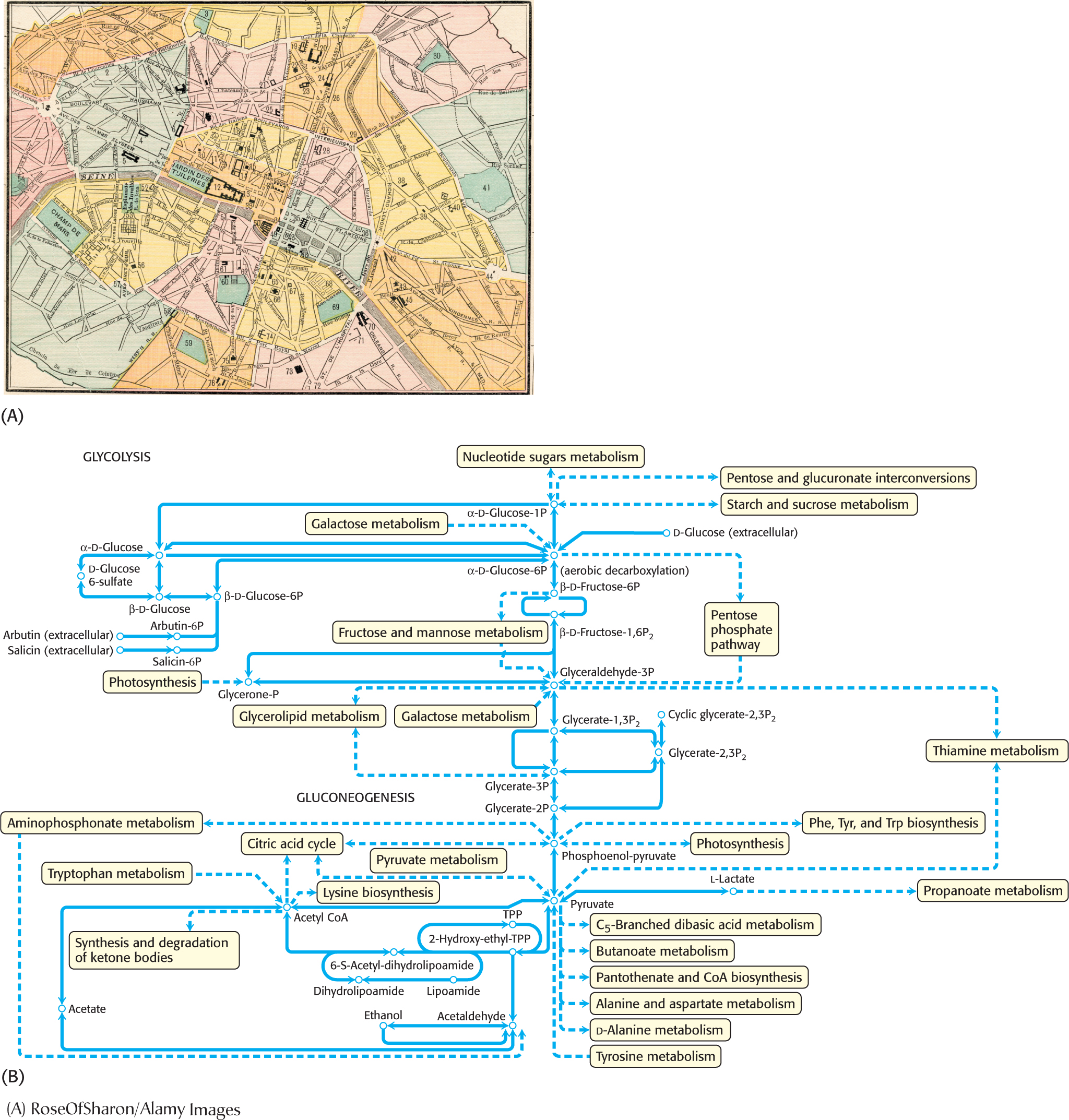
As important as catalysis is to the functioning of a cell, catalysis alone is not sufficient. The vast array of reaction pathways also need to be regulated so that the biochemical pathways function in a coherent fashion. Figure 7.7A is a street map of part of Paris. Every day, hundreds of thousands of vehicles travel these streets. Although the streets of Paris are notoriously difficult to navigate by car, imagine how much more difficult navigation would be without stop signs, stoplights, and traffic police. The metabolic map shown in Figure 7.7B shows that metabolic traffic also is immensely complex, and, like street traffic, metabolic traffic requires regulation. An effective means of regulating metabolic pathways is to regulate enzyme activity. Enzymes that regulate the flux of biochemicals through metabolic pathways are called allosteric enzymes, for reasons to be discussed shortly. Key features of allosteric enzymes include the regulation of catalytic activity by environmental signals, including the final product of the metabolic pathway regulated by the enzyme; kinetics that are more complex than those of Michaelis–
Allosteric Enzymes Are Regulated by Products of the Pathways Under Their Control
Let us begin our consideration of allosteric enzymes by imagining what control processes would be useful for the efficient functioning of metabolic pathways.
We begin by examining a hypothetical metabolic pathway:

There are five reactions in this metabolic pathway, catalyzed by five distinct enzymes, denoted e1 through e5. As is typical in real metabolic situations, the end product F is needed in limited amounts and cannot be stored. The initial reactant A is valuable and should be conserved unless F is needed. Finally, B, C, D, and E have no biological roles except as chemical intermediates in the synthesis of F. This last condition means that the first reaction, A → B, is the committed step in this pathway; after this reaction has taken place, B is committed to conversion into F. How can the production of F be regulated to meet the cellular requirements without making more than is needed?
In the simplest situation, when sufficient F is present, F can bind reversibly to e1, the enzyme catalyzing the committed step, and inhibit the reaction, an effect called feedback inhibition.
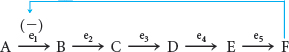
Feedback inhibition is a common means of biochemical regulation. Feedback inhibitors usually bear no structural resemblance to the substrate or the product of the enzyme that they inhibit. Moreover, feedback inhibitors do not bind at the active site but rather at a distinct regulatory site on the allosteric enzyme. Allosteric (from the Greek allos, meaning “other,” and stereos, meaning “structure”) enzymes are sonamed because they are regulated by molecules that bind to sites other than the active site. Allosteric enzymes always catalyze the committed step of metabolic pathways.
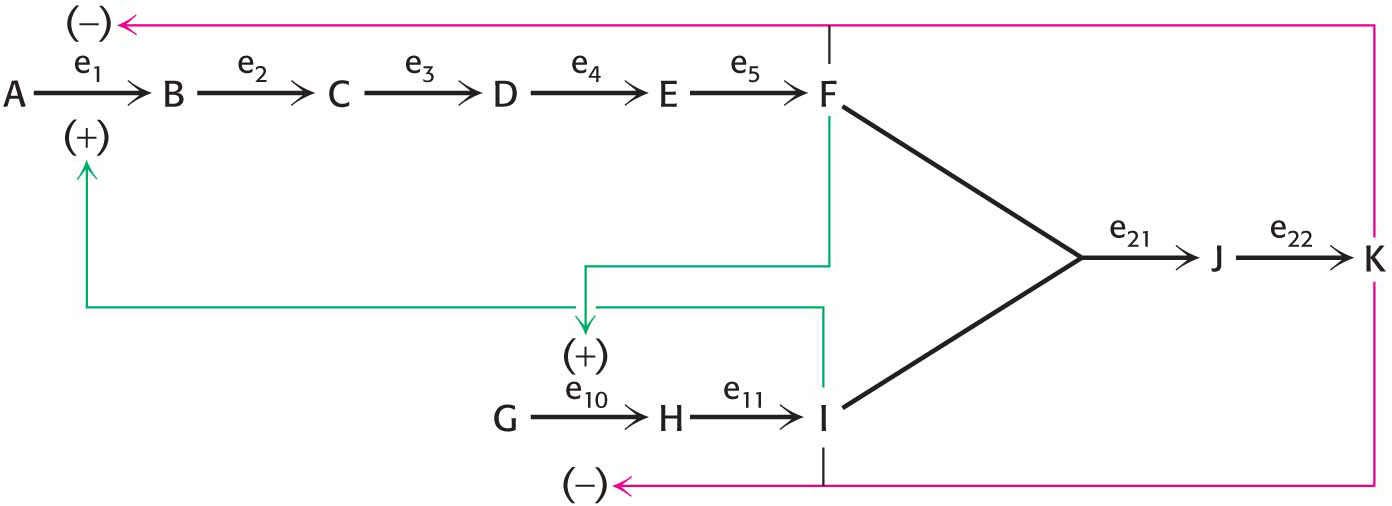
Another level of complexity is required when metabolic pathways must communicate with one another. Consider the pathway shown in Figure 7.8 for the synthesis of K from two separate pathways producing F and I. How can these pathways be coordinated to produce the appropriate amount of K? Compound K might inhibit enzymes e1 and e10 by feedback inhibition, inasmuch as these two enzymes control the committed step for each of the two required pathways. But, in this case, a second wasteful situation could arise: if more F were produced than I, some of the F molecules would be wasted because there would not be enough I to bind to F. Thus, how might the concentrations of F and I be balanced? Compound F could stimulate e10 but inhibit e1 to balance the amount of I with F. Likewise, compound I could inhibit e10 but stimulate e1. Thus, allosteric enzymes can recognize inhibitor molecules as well as stimulatory molecules.
Allosterically Regulated Enzymes Do Not Conform to Michaelis–Menten Kinetics
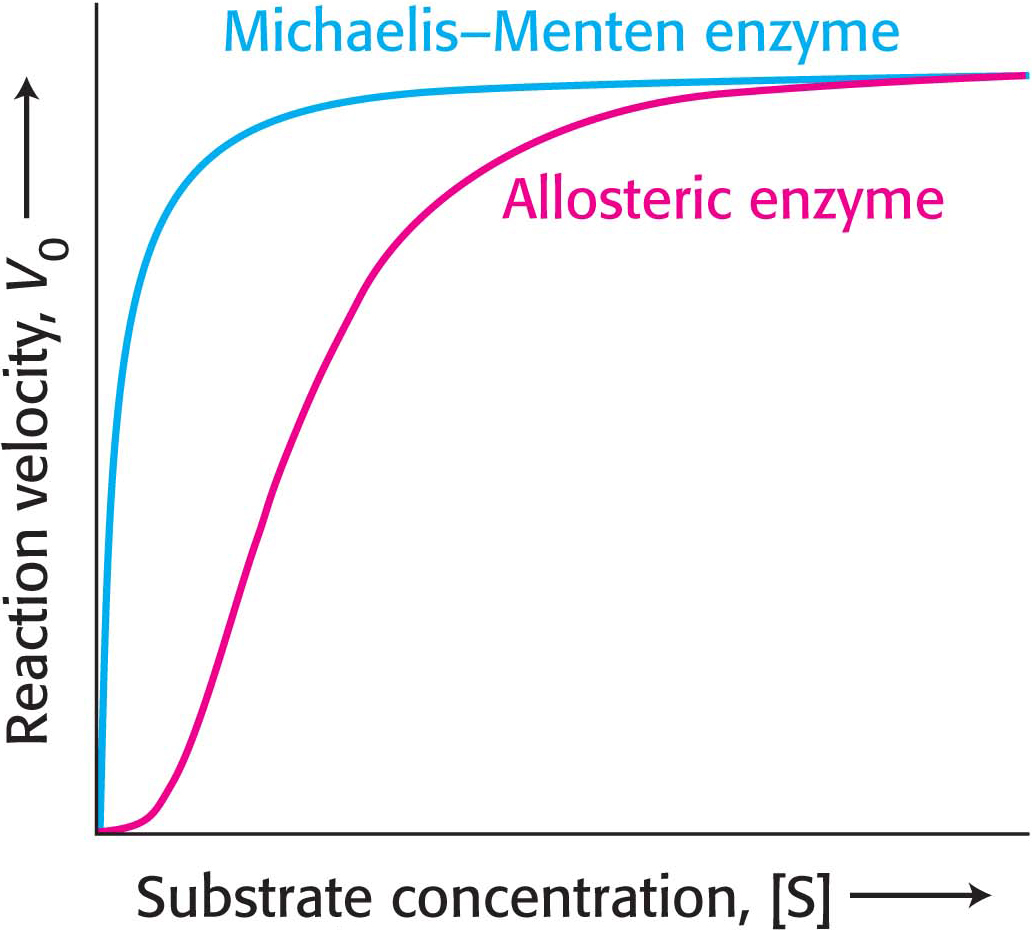
Allosteric enzymes are distinguished by their response to changes in substrate concentration in addition to their susceptibility to regulation by other molecules. A typical velocity-
Allosteric Enzymes Depend on Alterations in Quaternary Structure
Thus far, we know two properties that are unique to allosteric enzymes: (1) regulation of catalytic activity and (2) sigmoidal kinetics. Is there a relation between these two unique characteristics of allosteric enzymes? Indeed there is, and the relation is best elucidated by first examining a model for how sigmoidal kinetics might be displayed.
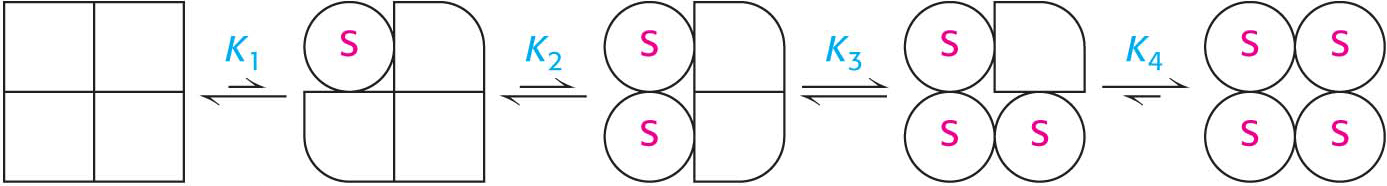
We will consider one possible model, called the concerted model or the MWC model after Jacques Monod, Jefferies Wyman, and Jean-
How can we use this information to explain the sigmoidal nature of allosteric kinetics? Consider a population of allosteric enzymes with each enzyme containing four active sites on four subunits. Because it is more stable, most of the enzymes will be in the T state. The binding of S to the T form is difficult; thus, there will be little activity at low substrate concentrations (Figure 7.10). However, if the substrate concentration is increased, eventually enough S will be present so that, when a relaxed form of the enzyme spontaneously appears, S will bind to it. Because of the symmetry rule, if one S binds to the R form, all of the four potential active sites become trapped in the R form. Consequently, the next S to bind the enzyme will not have to unproductively collide with the many T forms, because R forms of the enzymes are accumulating owing to the binding of the initial S to the enzyme. The binding of substrate disrupts the T ⇋ R equilibrium in favor of R. This behavior is called cooperativity and accounts for the sharp increase in V0 of the velocity-
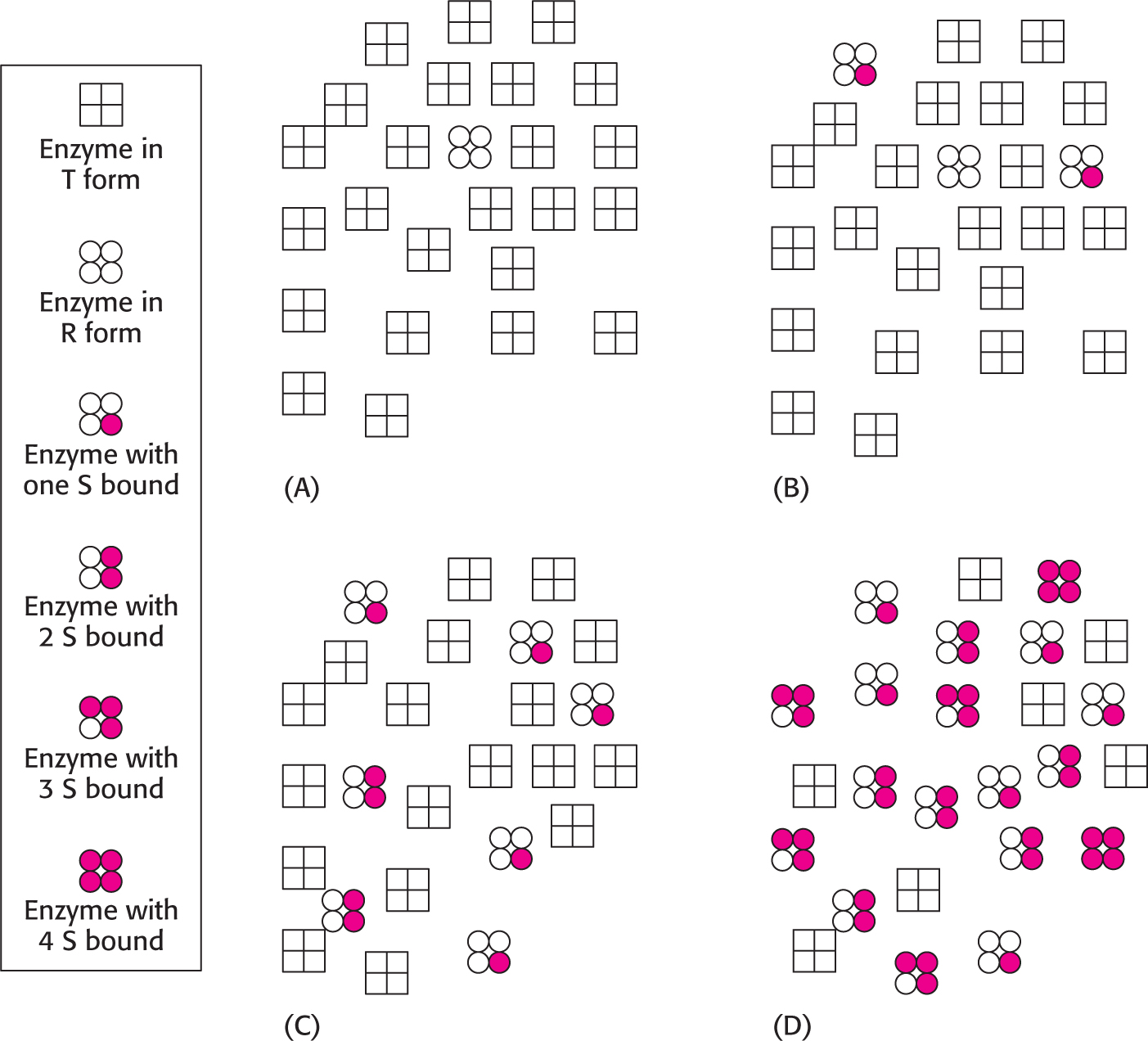
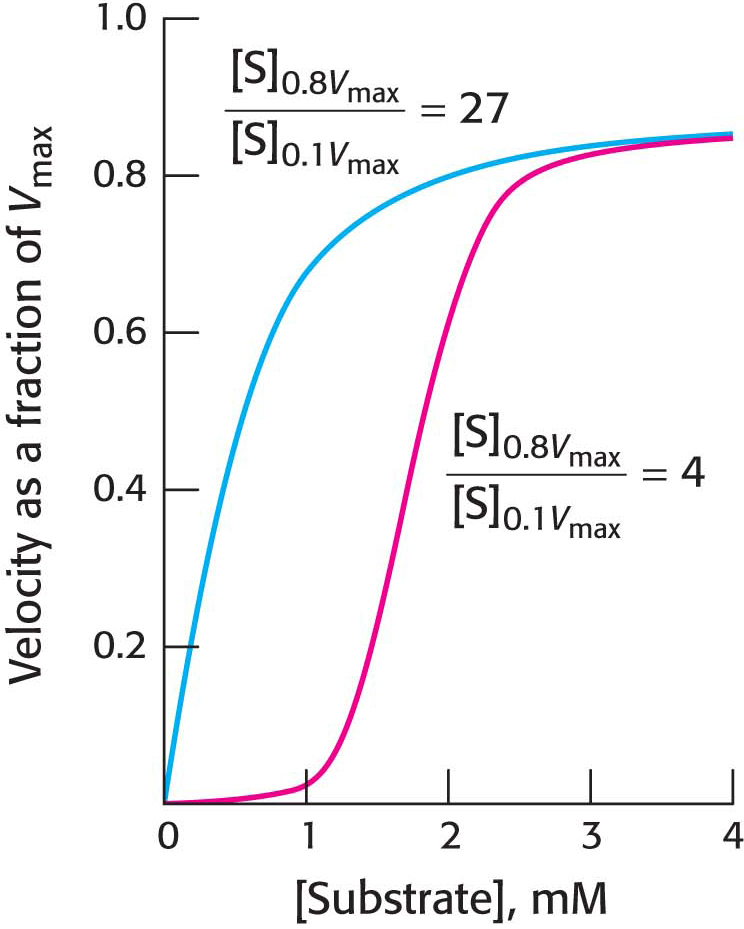
What is the physiological significance of cooperativity, seen as a sigmoidal kinetics? Allosteric enzymes transition from a less active state to a more active state within a narrow range of substrate concentration. The benefit of this behavior is illustrated in Figure 7.11, which compares the kinetics of a Michaelis–
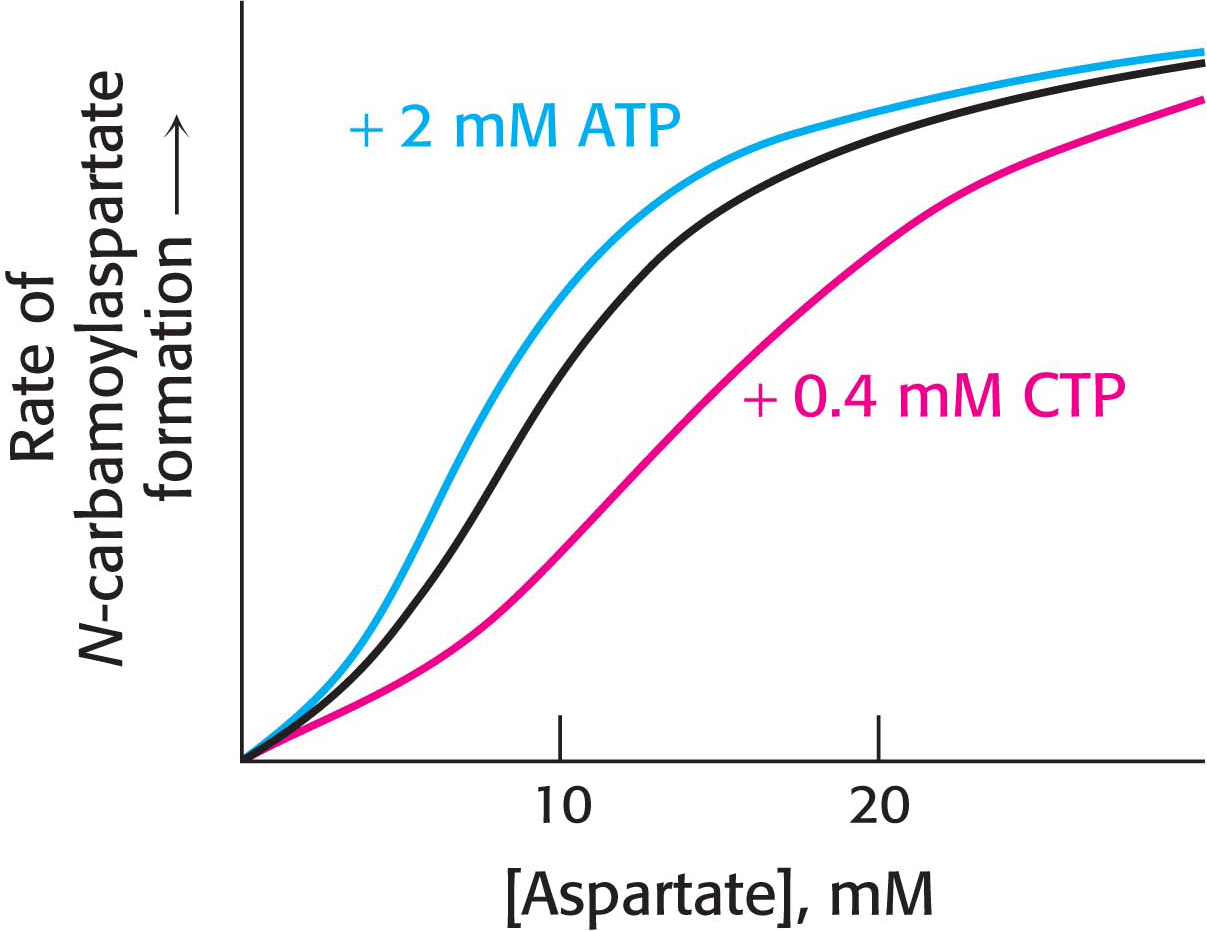
Regulator Molecules Modulate the T ⇋ R Equilibrium
How can we explain the signal-
QUICK QUIZ 2
What would be the effect of a mutation in an allosteric enzyme that resulted in a T/R ratio of 0?
All of the enzyme would be in the R form all of the time. There would be no cooperativity. The kinetics would look like that of a Michaelis–
It is all but impossible to overestimate the importance of allosteric enzymes in biological systems. They respond immediately and specifically to chemical signals produced elsewhere in the cell. They are receivers and transducers of chemical information, allowing cross talk between metabolic pathways, not only within the cell, but also between cells and organs. The complexity of biological systems is due to the sensing of the environment by allosteric enzymes.
The Sequential Model Also Can Account for Allosteric Effects
In the concerted model, an allosteric enzyme can exist in only two states, T and R; no intermediate or hybrid states are allowed. An alternative model posits that the subunits of the allosteric enzyme undergo sequential changes in structure. The binding of substrate to one site influences the substrate binding to neighboring sites without necessarily inducing a transition encompassing the entire enzyme (Figure 7.13). The sequential model more readily accommodates negative cooperativity, in which the binding of one substrate decreases the affinity of other sites for the substrate. The results of studies on a number of allosteric proteins suggest that many behave according to some combination of the sequential and concerted models.
 CLINICAL INSIGHT
CLINICAL INSIGHTLoss of Allosteric Control May Result in Pathological Conditions
The importance of the allosteric control of enzymes can be seen in the pathway leading to the joint disease gout. In this disease, an excess of urate crystallizes in the fluid and lining of the joints, resulting in painful inflammation when cells of the immune system engulf the sodium urate crystals (Figure 7.14). Urate is a final product of the degradation of purines, the base components of the adenine and guanine nucleotides of DNA and RNA.
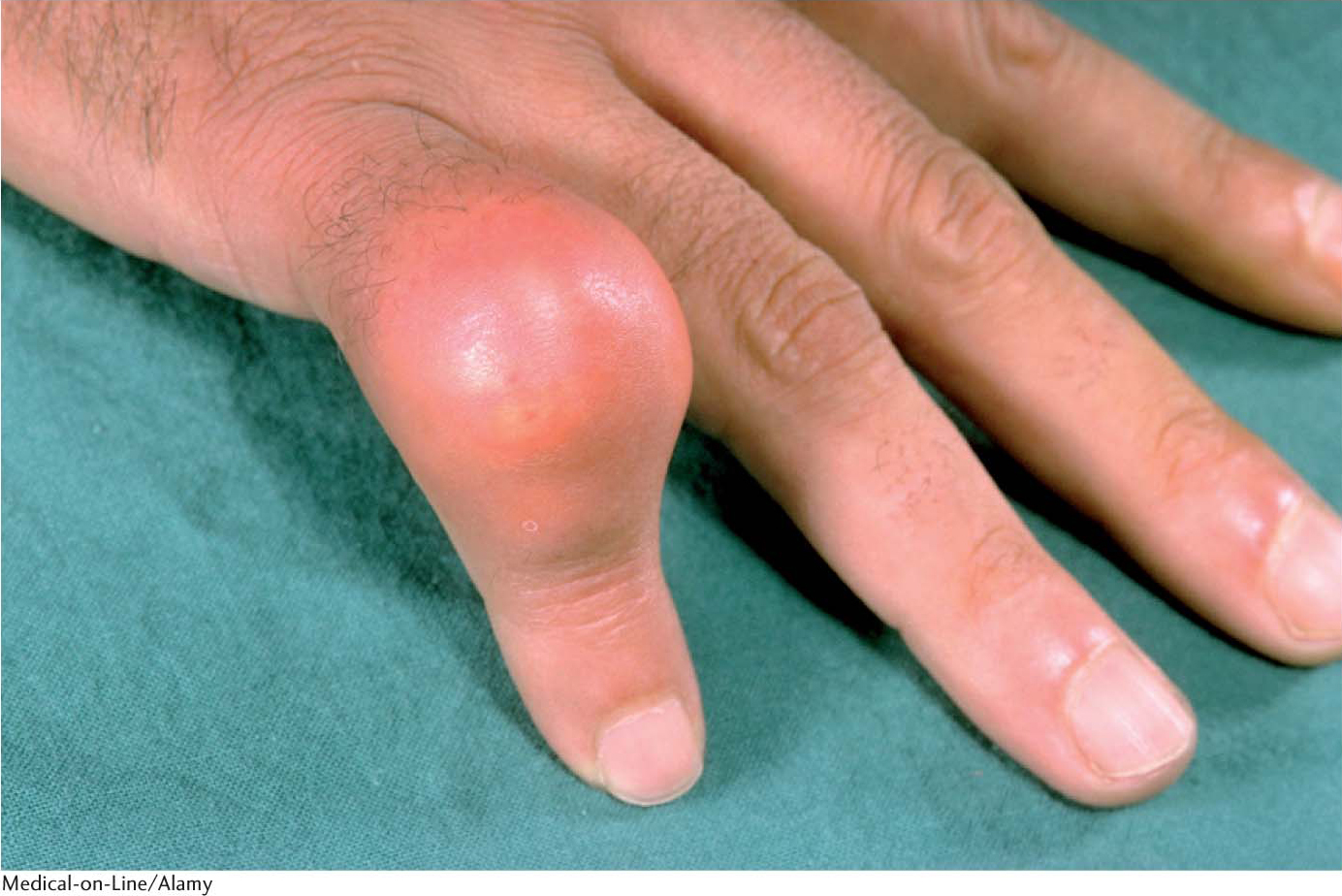
Although gout can be caused by a number of metabolic deficiencies (Chapter 31), one interesting cause is the loss of allosteric regulation by an important enzyme in purine synthesis. Phosphoribosylpyrophosphate synthetase (PRS) catalyzes the synthesis of phosphoribosylpyrophosphate (PRPP), which is a vital precursor for all nucleotide synthesis:
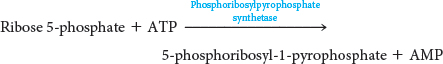
PRS is normally feedback inhibited by purine nucleotides. However, in certain people, the regulatory site has undergone a mutation that renders PRS insensitive to feedback inhibition. The catalytic activity of PRS is unaffected and, in some cases, is increased, leading to a glut of purine nucleotides, which are converted into urate. The excess urate accumulates and causes gout.