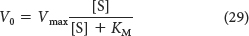APPENDIX: Derivation of the Michaelis–Menten Equation
As already discussed, the key feature of the Michaelis–
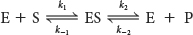
An enzyme E combines with substrate S to form an ES complex, with a rate constant k1. The ES complex has two possible fates. It can dissociate to E and S, with a rate constant k−1, or it can proceed to form product P, with a rate constant k2. The ES complex can also be re-
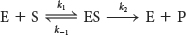
We want an expression that relates the velocity of catalysis to the concentrations of substrate and enzyme and the rates of the individual steps. Our starting point is that the catalytic velocity is equal to the product of the concentration of the ES complex and k2:

Now we need to express [ES] in terms of known quantities. The rates of formation and breakdown of ES are given by
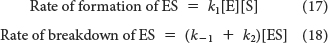
To simplify matters further, we use the steady-
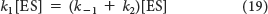
By rearranging equation 19, we obtain
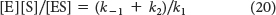
Equation 20 can be simplified by defining a new constant, KM, called the Michaelis constant:
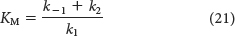
Note that KM has the units of concentration and is independent of enzyme and substrate concentrations.
Inserting equation 21 into equation 20 and solving for [ES] yields

Now, let us examine the numerator of equation 22. Because the substrate is usually present at much higher concentration than the enzyme, the concentration of uncombined substrate [S] is very nearly equal to the total substrate concentration. The concentration of uncombined enzyme [E] is equal to the total enzyme concentration [E]T minus the concentration of the ES complex:

Substituting this expression for [E] in equation 22 gives
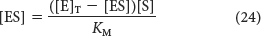
Solving equation 24 for [ES] gives
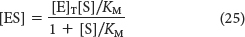
or
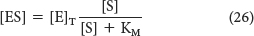
By substituting this expression for [ES] into equation 16, we obtain
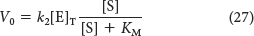
The maximal velocity Vmax is attained when the catalytic sites on the enzyme are saturated with substrate—

Substituting equation 28 into equation 27 yields the Michaelis–