8.2 Enzyme Activity Can Be Modulated by Temperature, pH, and Inhibitory Molecules
✓ 6 List environmental factors that affect enzyme activity, and describe how these factors exert their effects on enzymes.
Regardless of which mechanism or mechanisms are employed by an enzyme to catalyze a reaction, the rate of catalysis is affected by the same environmental parameters that affect all chemical reactions, such as temperature and pH. Moreover, some chemicals that interact specifically with the elaborate three-
Temperature Enhances the Rate of Enzyme-Catalyzed Reactions
As the temperature rises, the rate of most reactions increases. The rise in temperature increases the Brownian motion of the molecules, which makes interactions between an enzyme and its substrate more likely. For most enzymes, there is a temperature at which the increase in catalytic activity ceases and there is a precipitous loss of activity (Figure 8.1). What is the basis of this loss of activity? Recall from Chapter 4 that proteins have a complex three-
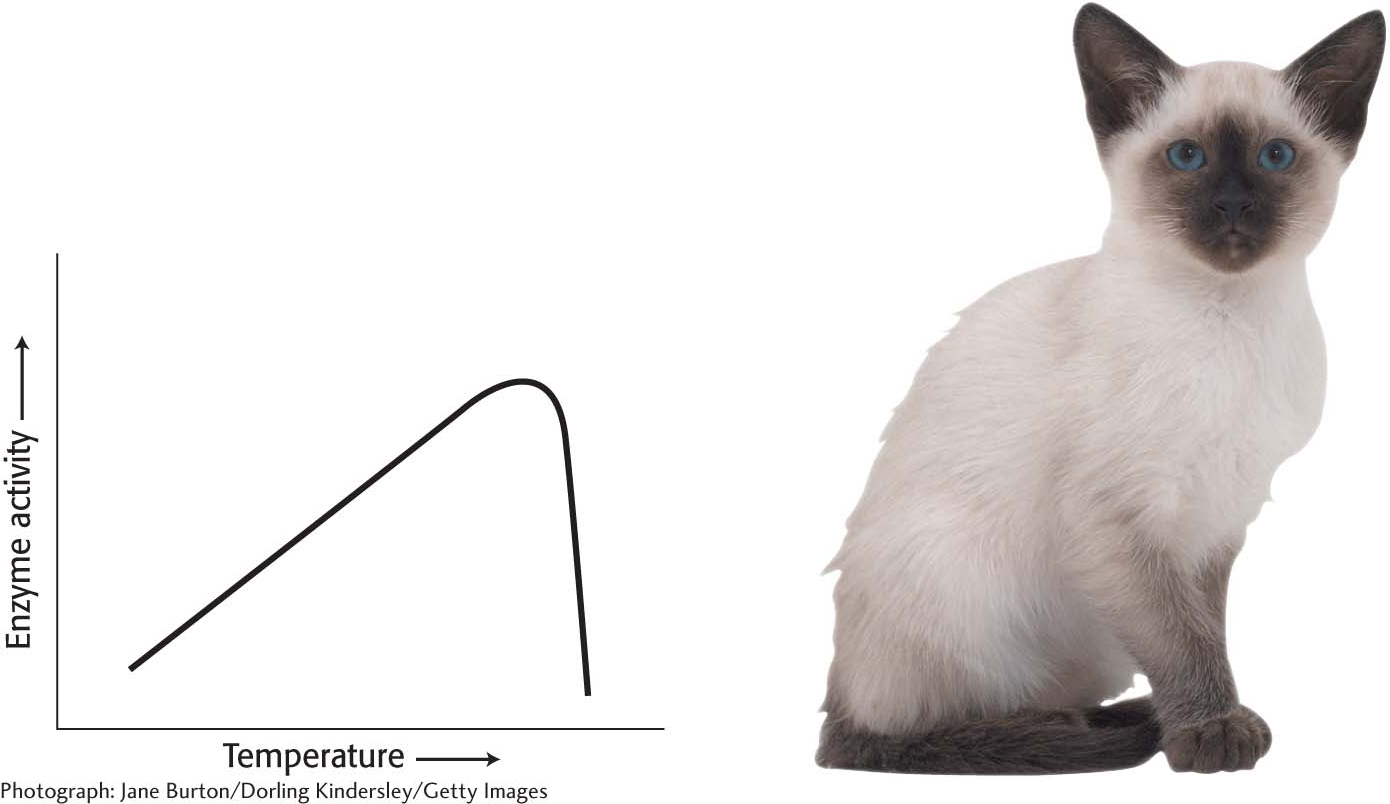

In organisms such as ourselves that maintain a constant body temperature (endotherms), the effect of outside temperature on enzyme activity is minimized. However, in organisms that assume the temperature of the ambient environment (ectotherms), temperature is an important regulator of biochemical and, indeed, biological activity. Lizards, for instance, are most active in warmer temperatures and relatively inactive in cooler temperatures, a behavioral manifestation of biochemical activity (Figure 8.2). Although endotherms are not as sensitive to ambient temperature as ectotherms, slight tissue temperature alterations are sometimes important. For instance, when athletes “warm-
Some organisms, such as thermophilic archaea, can live at temperatures of 80°C or higher, temperatures that would denature most proteins. The proteins in these organisms have evolved to be very resistant to thermal denaturation (Figure 8.3).

Most Enzymes Have an Optimal pH
Enzyme activity also often varies with pH, the H+ concentration of the environment. The activity of most enzymes displays a bell-
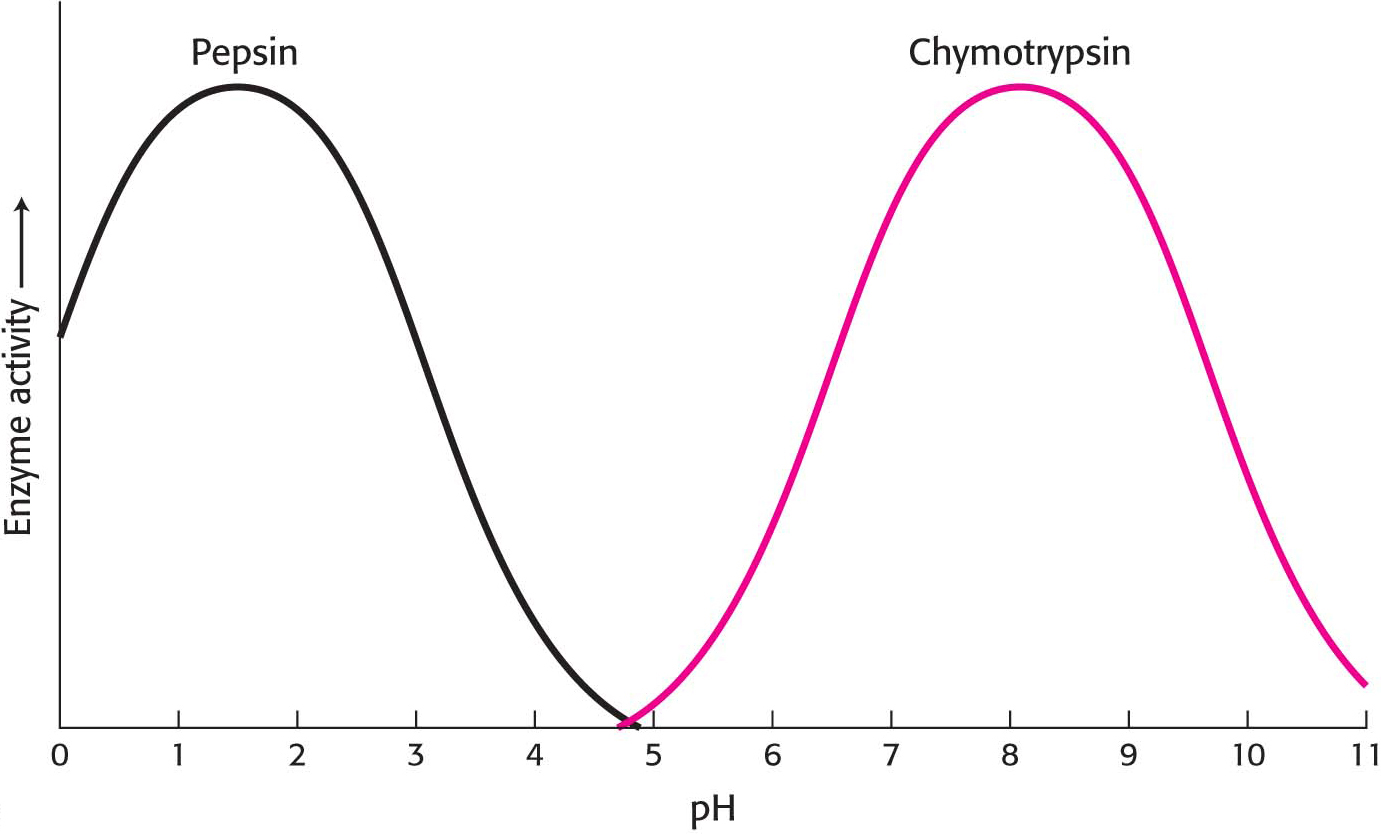
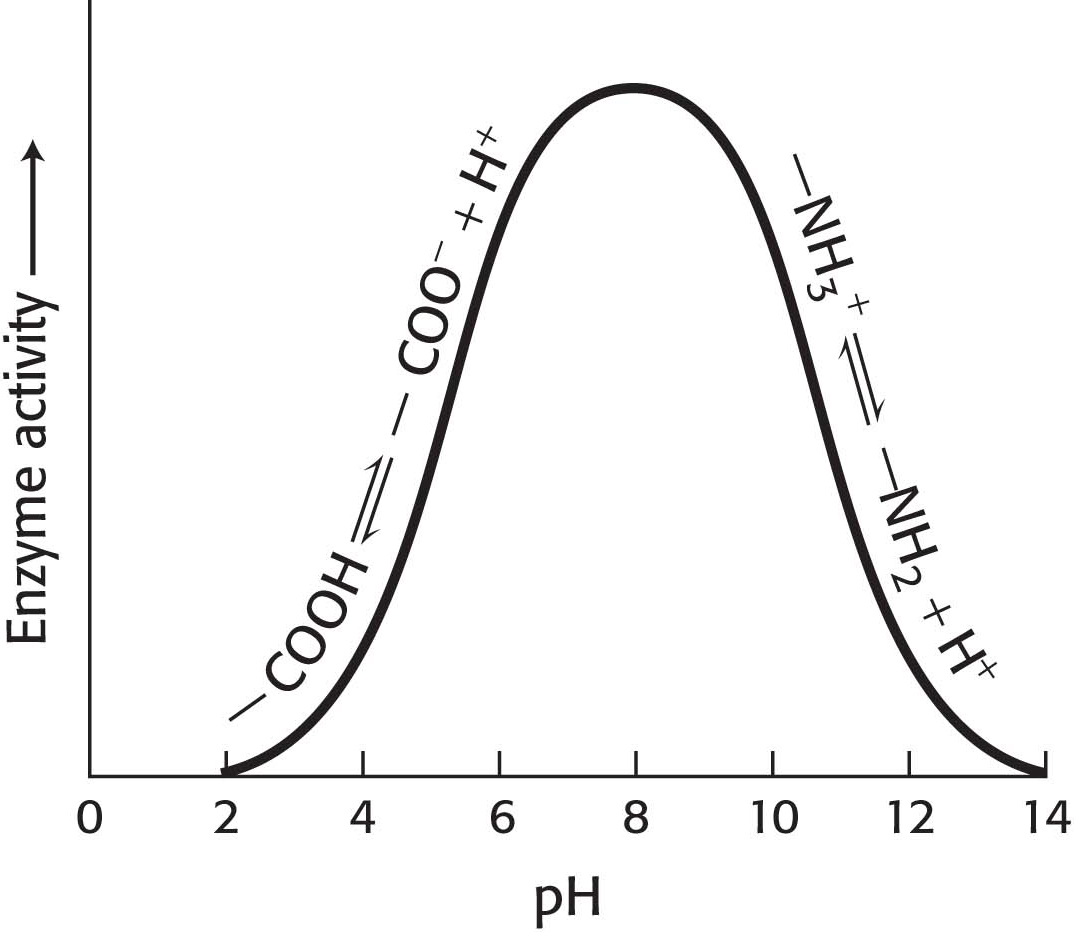
How can we account for the pH effect on enzyme activity in regard to our understanding of enzymes in particular and proteins in general? Imagine an enzyme that requires the ionization of both a glutamic acid residue and a lysine residue at the active site for the enzyme to be functional. Thus, the enzyme would depend on the presence of a—
Is alteration of the pH of the cellular milieu ever used as a regulator device? The answer is yes, and a crucial enzyme in glucose metabolism in skeletal muscle provides an example. Phosphofructokinase (Chapter 16) controls the rate of metabolism of glucose under aerobic conditions (in the presence of oxygen) and under anaerobic conditions (in the absence of oxygen). A problem arises with the rapid processing of glucose in the absence of oxygen: the end product is lactic acid, which readily ionizes to lactate and a hydrogen ion. To prevent the muscle from becoming “pickled” by the high concentration of acid, the activity of phosphofructokinase decreases if the pH falls too drastically, which, in turn, reduces the metabolism of glucose to lactic acid. Phosphofructokinase is made up of multiple subunits, and the decrease in pH causes the subunits to dissociate, rendering the enzyme inactive and thus reducing lactic acid production.
Enzymes Can Be Inhibited by Specific Molecules
The activity of many enzymes can be inhibited by the binding of specific small molecules and ions. This means of enzyme inhibition serves as a major control mechanism in biological systems, typified by the regulation of allosteric enzymes. In addition, many drugs and toxic agents act by inhibiting enzymes. This type of enzyme inhibition is not usually the result of evolutionary forces, as it is for allosteric enzymes, but rather due to design of inhibitors by scientists or simple chance discovery of inhibitory molecules. Examining inhibition can be a source of insight into the mechanism of enzyme action: specific inhibitors can often be used to identify residues critical for catalysis. Transition-
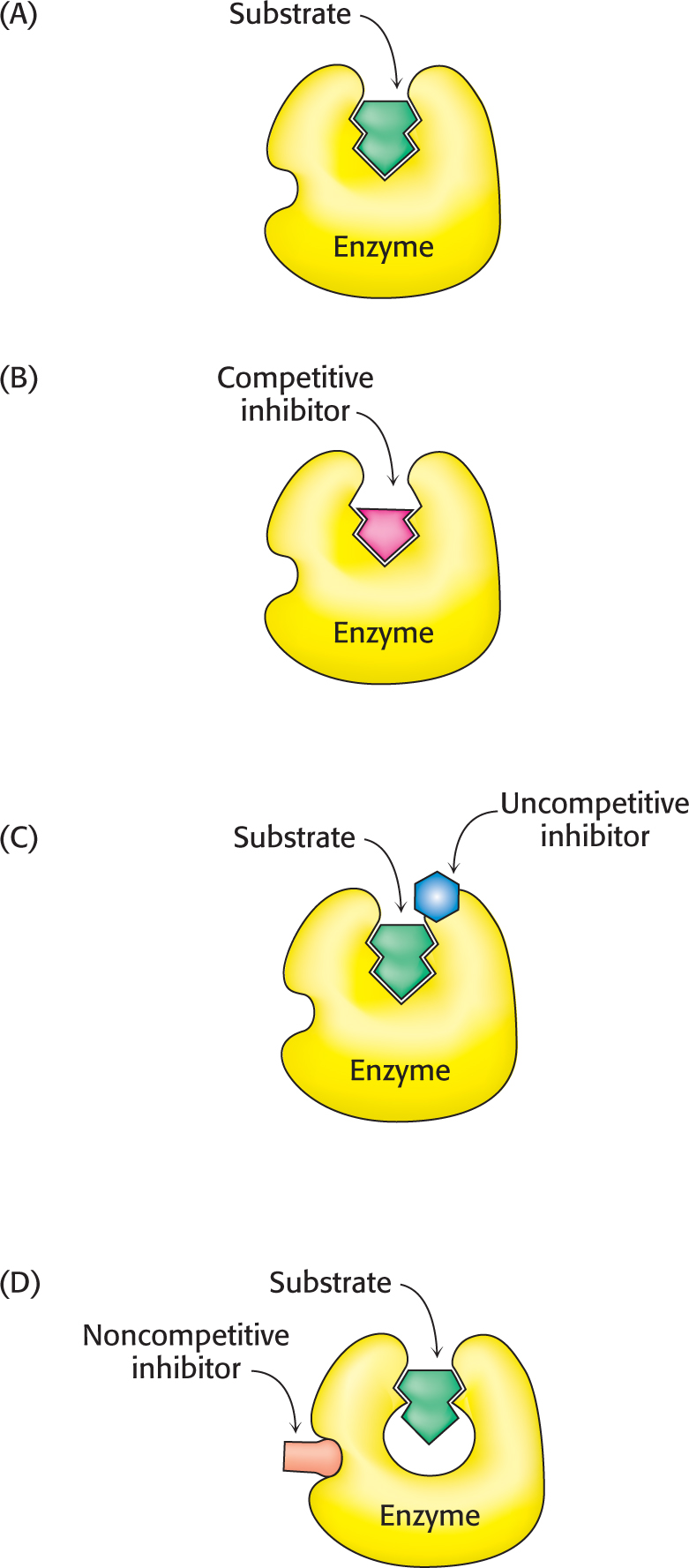
Enzyme inhibition can be either reversible or irreversible. We begin the investigation of enzyme inhibition by first examining reversible inhibition. In contrast with irreversible inhibition, reversible inhibition is characterized by rapid dissociation of the enzyme–
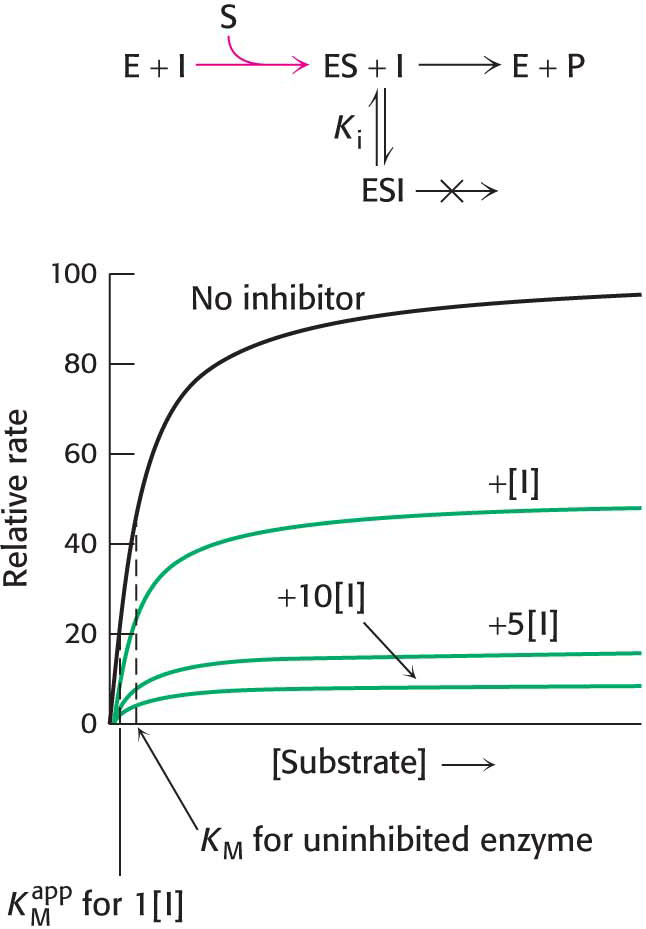
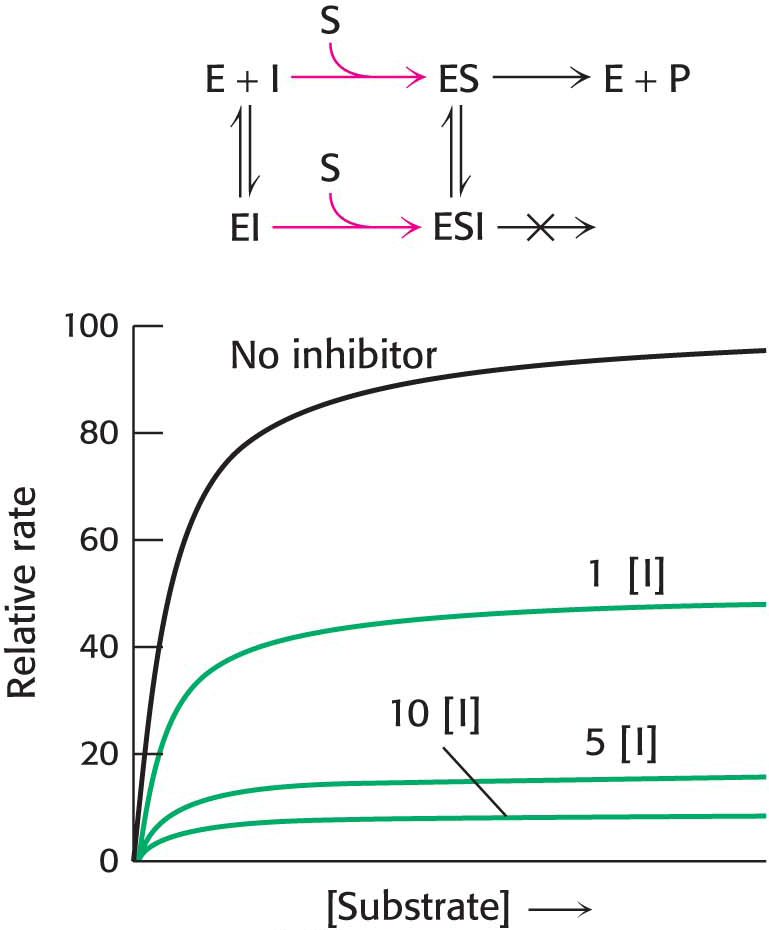
In competitive inhibition, the inhibitor resembles the substrate and binds to the active site of the enzyme (Figure 8.6B). The substrate is thereby prevented from binding to the same active site. An enzyme can bind substrate (forming an ES complex) or inhibitor (EI), but not both (ESI). A competitive inhibitor diminishes the rate of catalysis by reducing the proportion of enzyme molecules bound to a substrate. At any given inhibitor concentration, competitive inhibition can be relieved by increasing the substrate concentration. Under these conditions, the substrate “outcompetes” the inhibitor for the active site.
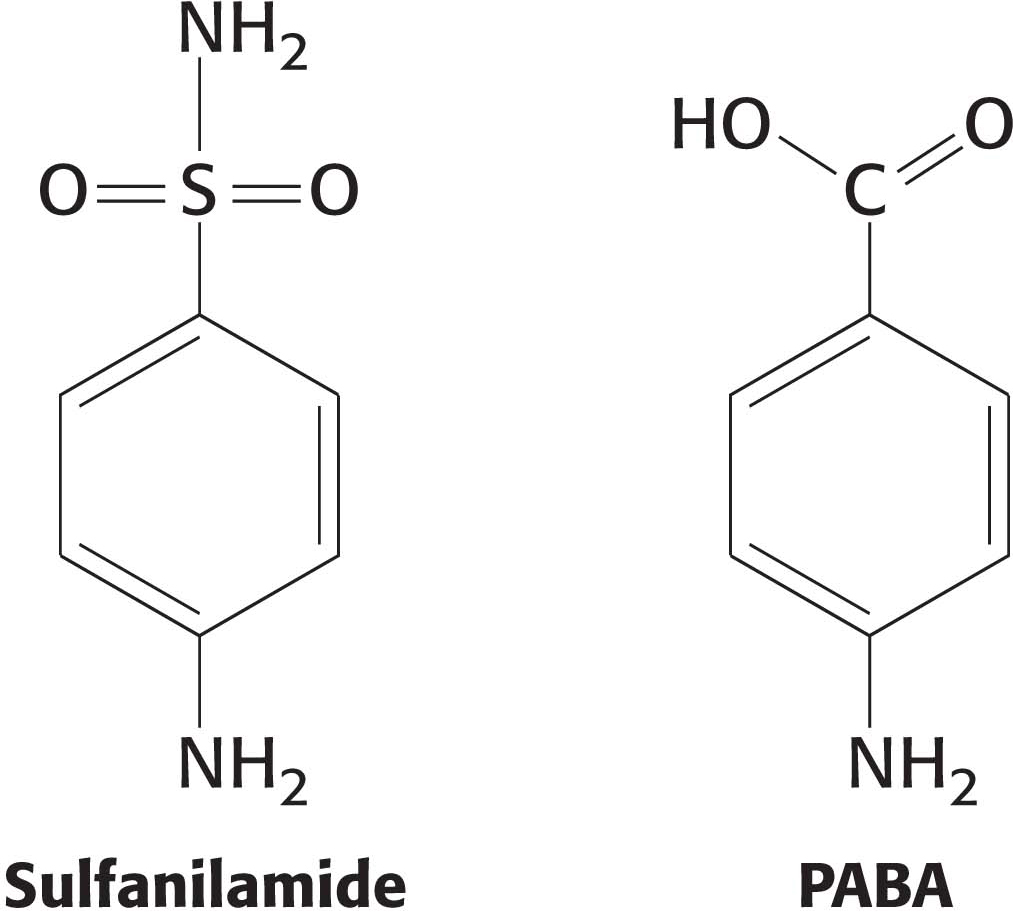
Some competitive inhibitors are useful drugs. One of the earliest examples was the use of sulfanilamide as an antibiotic. Sulfanilamide is an example of a sulfa drug, a sulfur-
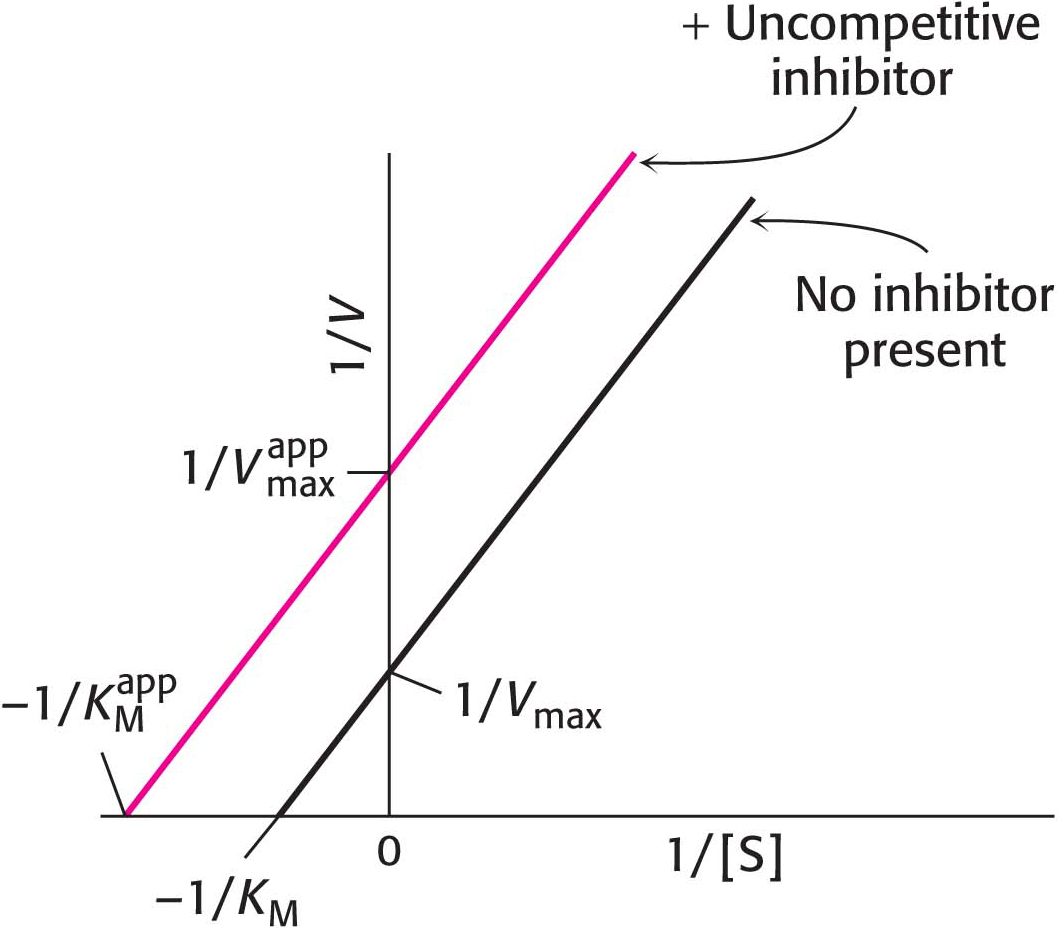
Uncompetitive inhibition is substrate-
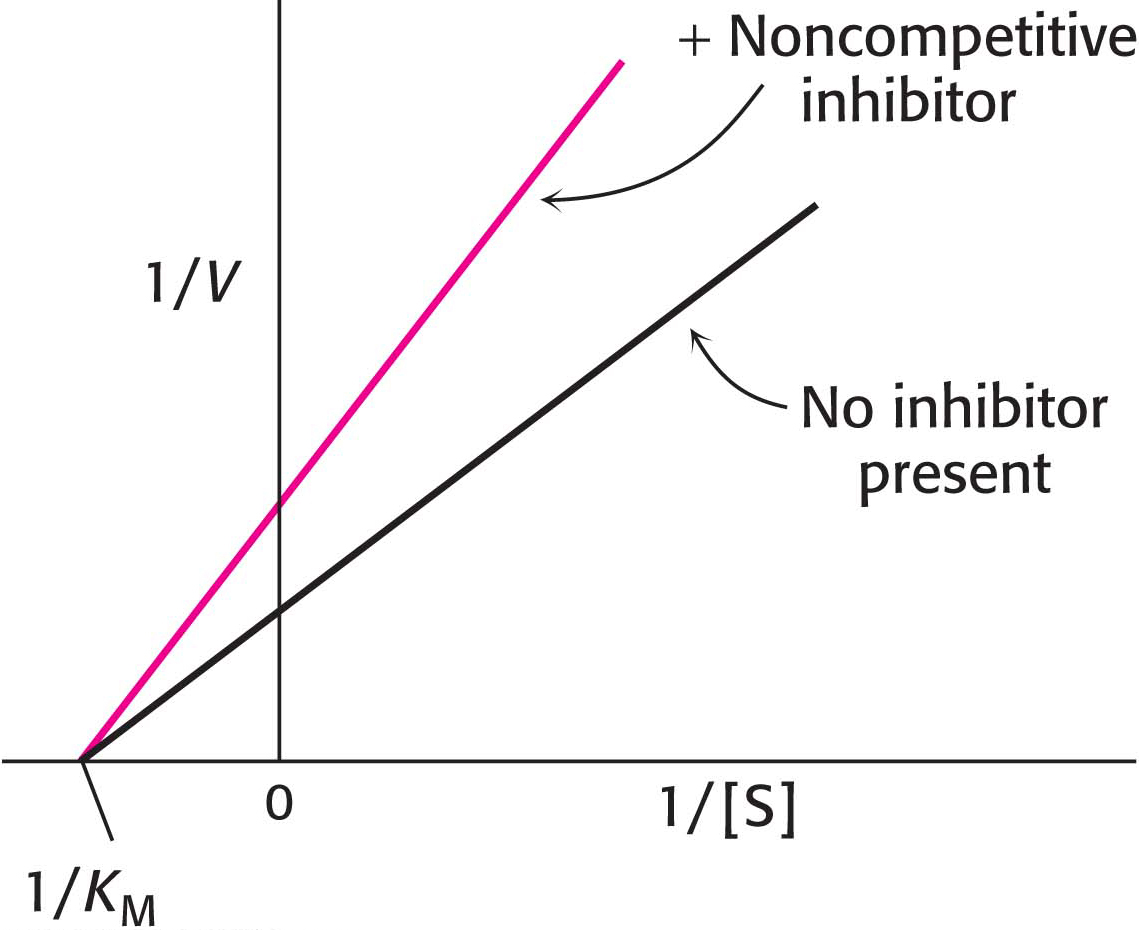
In noncompetitive inhibition, the inhibitor and substrate can bind simultaneously to an enzyme molecule at different binding sites (Figure 8.6D). A noncompetitive inhibitor acts by decreasing the overall number of active enzyme molecules rather than by diminishing the proportion of enzyme molecules that are bound to substrate. Noncompetitive inhibition, in contrast with competitive inhibition, cannot be overcome by increasing the substrate concentration. Doxycycline, an antibiotic, functions at low concentrations as a noncompetitive inhibitor of a bacterial proteolytic enzyme (collagenase). Inhibition of this enzyme prevents the growth and reproduction of bacteria that cause gum (periodontal) disease.
Reversible Inhibitors Are Kinetically Distinguishable
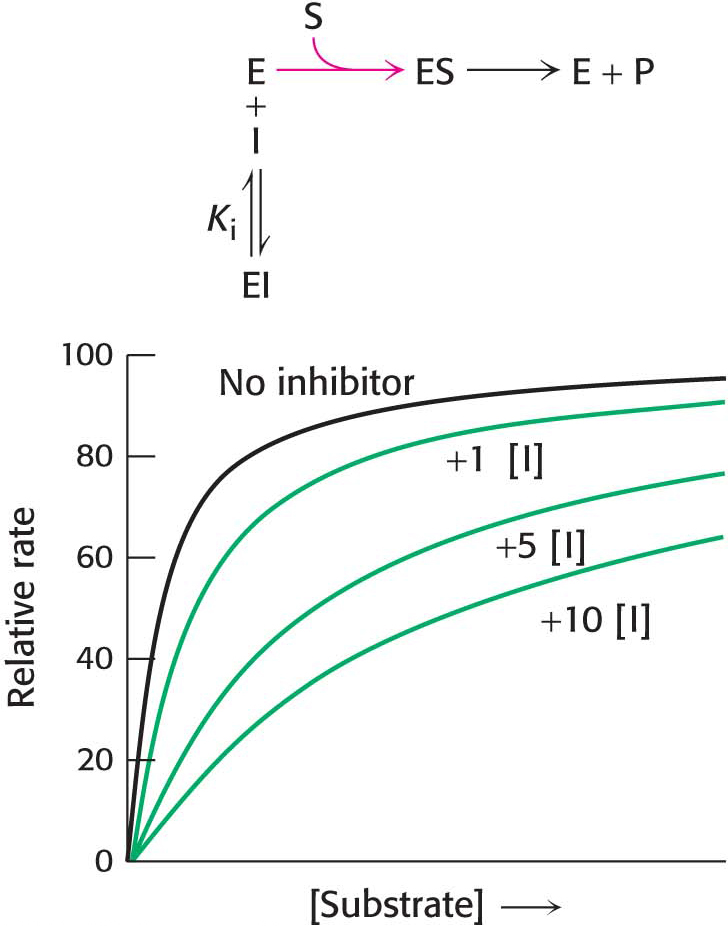
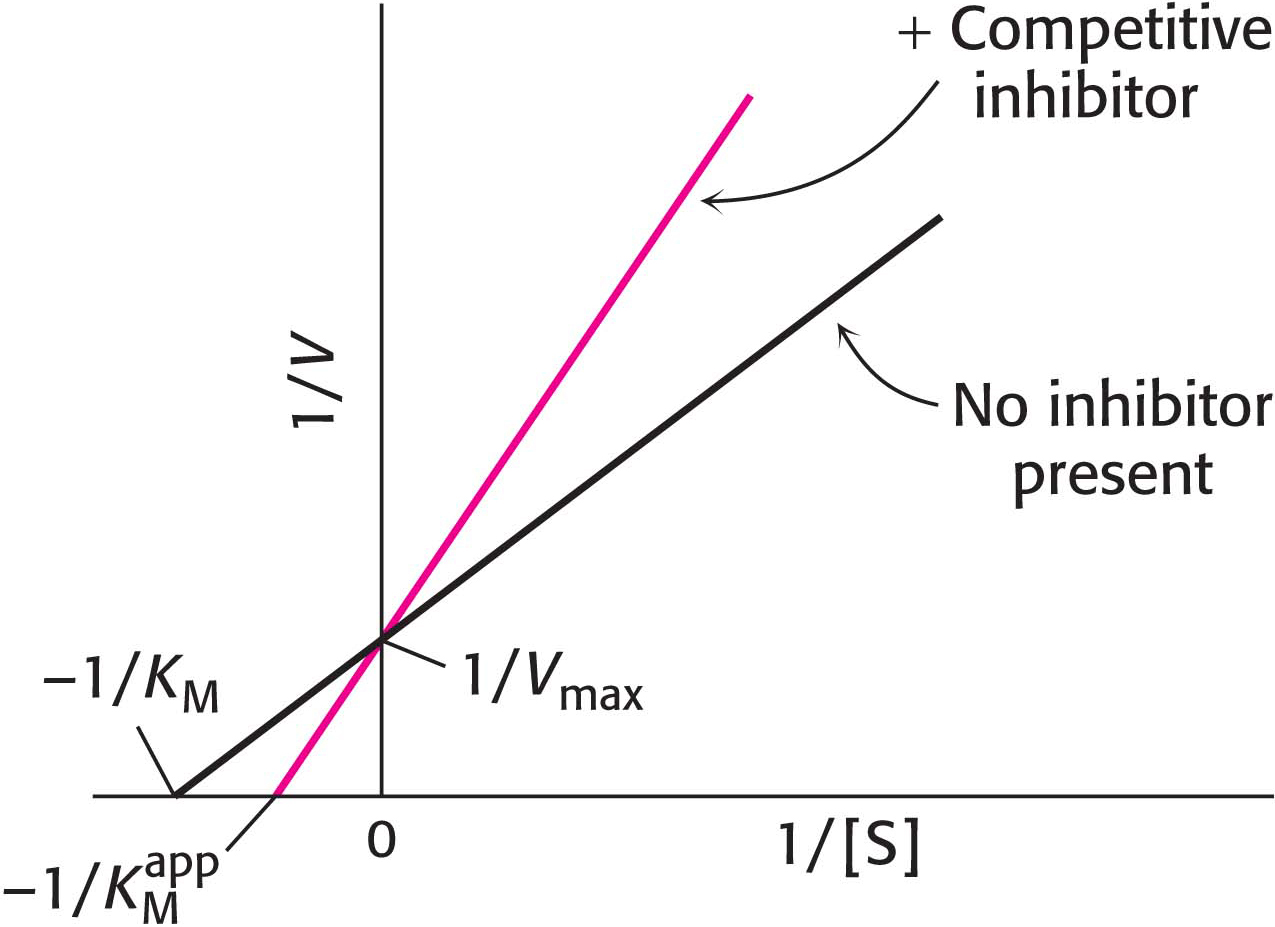
How can we determine whether a reversible inhibitor acts by competitive, uncompetitive, or noncompetitive inhibition? Let us consider only enzymes that exhibit Michaelis–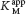 . In the presence of a competitive inhibitor, an enzyme will have the same Vmax as in the absence of an inhibitor. At a sufficiently high concentration, virtually all the active sites are filled by substrate, and the enzyme is fully operative. The more inhibitor present, the more substrate is required to displace it and reach Vmax.
. In the presence of a competitive inhibitor, an enzyme will have the same Vmax as in the absence of an inhibitor. At a sufficiently high concentration, virtually all the active sites are filled by substrate, and the enzyme is fully operative. The more inhibitor present, the more substrate is required to displace it and reach Vmax.
In uncompetitive inhibition, the inhibitor binds only to the ES complex. This enzyme–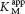 . Likewise, the value of Vmax is decreased to a new value called
. Likewise, the value of Vmax is decreased to a new value called 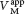 .
.
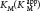 is lowered, becoming smaller as more inhibitor is added.
is lowered, becoming smaller as more inhibitor is added.In noncompetitive inhibition (Figure 8.9), a substrate can bind to the enzyme–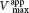 , whereas the value of KM is unchanged. Why is Vmax lowered although KM remains unchanged? In essence, the inhibitor simply lowers the concentration of functional enzyme. The resulting solution behaves as a more dilute solution of enzyme. Noncompetitive inhibition cannot be overcome by increasing the substrate concentration.
, whereas the value of KM is unchanged. Why is Vmax lowered although KM remains unchanged? In essence, the inhibitor simply lowers the concentration of functional enzyme. The resulting solution behaves as a more dilute solution of enzyme. Noncompetitive inhibition cannot be overcome by increasing the substrate concentration.
Double-
In uncompetitive inhibition (Figure 8.11), the inhibitor combines only with the enzyme–
In noncompetitive inhibition (Figure 8.12), the inhibitor can combine with either the enzyme or the enzyme–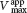 , and so the intercept on the vertical axis is increased. The slope when the inhibitor is present, which is equal to
, and so the intercept on the vertical axis is increased. The slope when the inhibitor is present, which is equal to 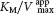 , is larger by the same factor. In contrast with Vmax, KM is not affected by pure noncompetitive inhibition.
, is larger by the same factor. In contrast with Vmax, KM is not affected by pure noncompetitive inhibition.
Irreversible Inhibitors Can Be Used to Map the Active Site
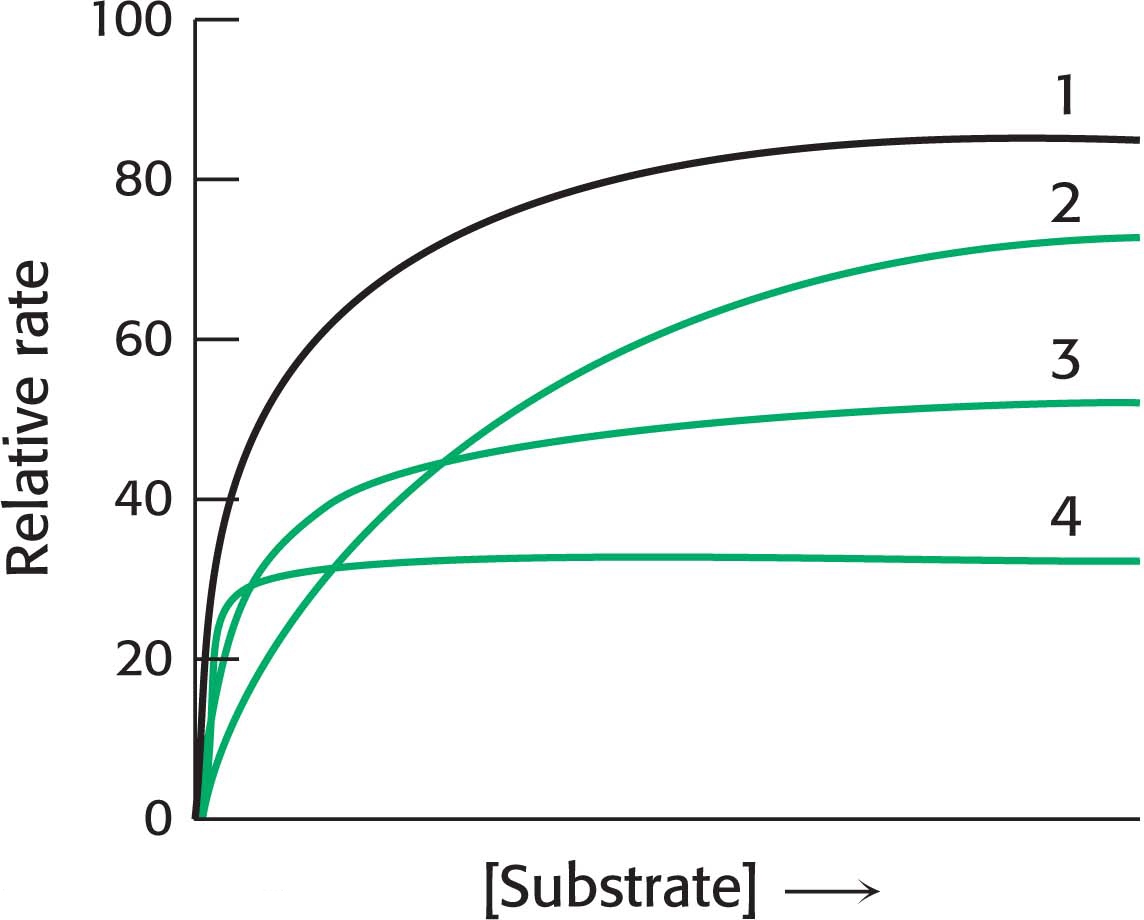
QUICK QUIZ 1
In the following graph, identify the curve that corresponds to each of the following conditions: no inhibition, competitive inhibition, noncompetitive inhibition, uncompetitive inhibition.
Curve 1, no inhibition; curve 2, competitive inhibition; curve 3, noncompetitive inhibition; curve 4, uncompetitive inhibition.
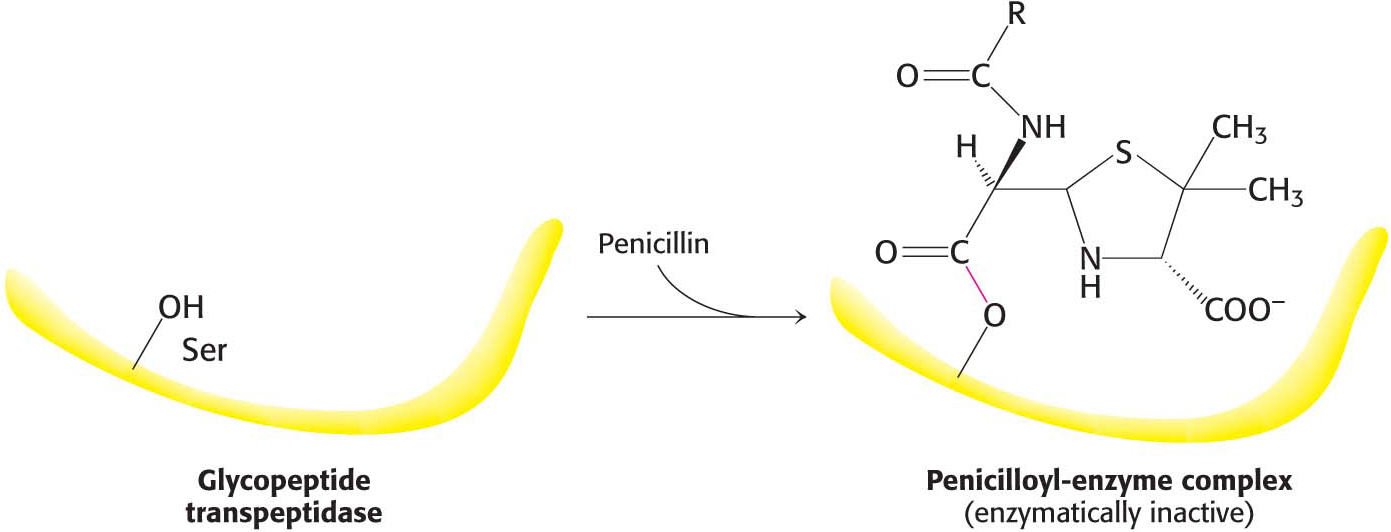
Whereas a reversible inhibitor will both bind to an enzyme and dissociate from it rapidly, an irreversible inhibitor dissociates very slowly from its target enzyme because it has become tightly bound to the enzyme, either covalently or noncovalently. Some irreversible inhibitors are important drugs. Penicillin acts by covalently modifying the enzyme transpeptidase, thereby preventing the synthesis of bacterial cell walls and thus killing the bacteria. Aspirin acts by covalently modifying the enzyme cyclooxygenase (the same enzyme inhibited by ibuprofen), reducing the synthesis of inflammatory signals.
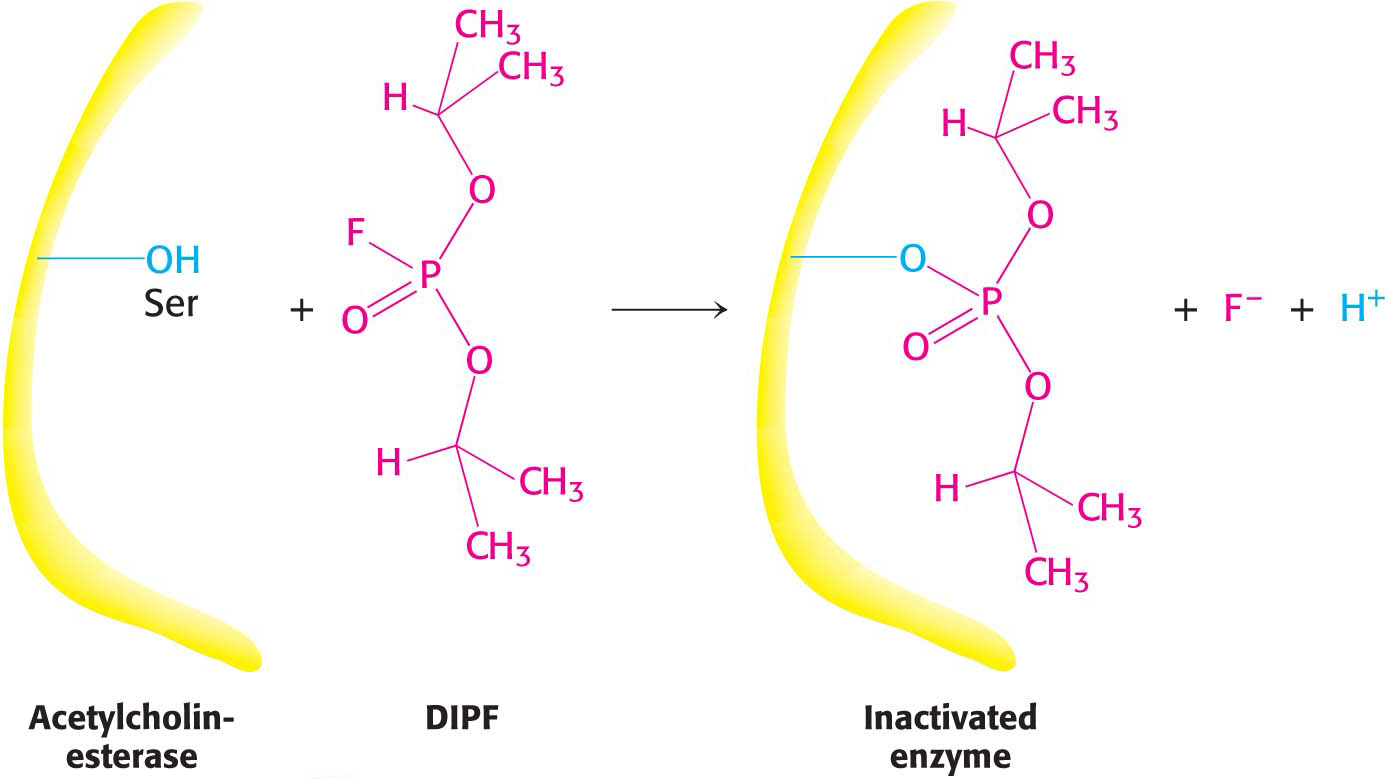
Irreversible inhibitors that covalently bind to an enzyme are tools for elucidating the mechanism of enzymes. The first step in determining the chemical mechanism of an enzyme is to determine which functional groups are required for enzyme activity. Irreversible inhibitors modify functional groups, which can then be identified. If treatment with an irreversible inhibitor results in a loss of enzyme activity, then this loss suggests that the modified group is required for enzyme activity. Irreversible inhibitors can be assorted into four categories: group-
Group-
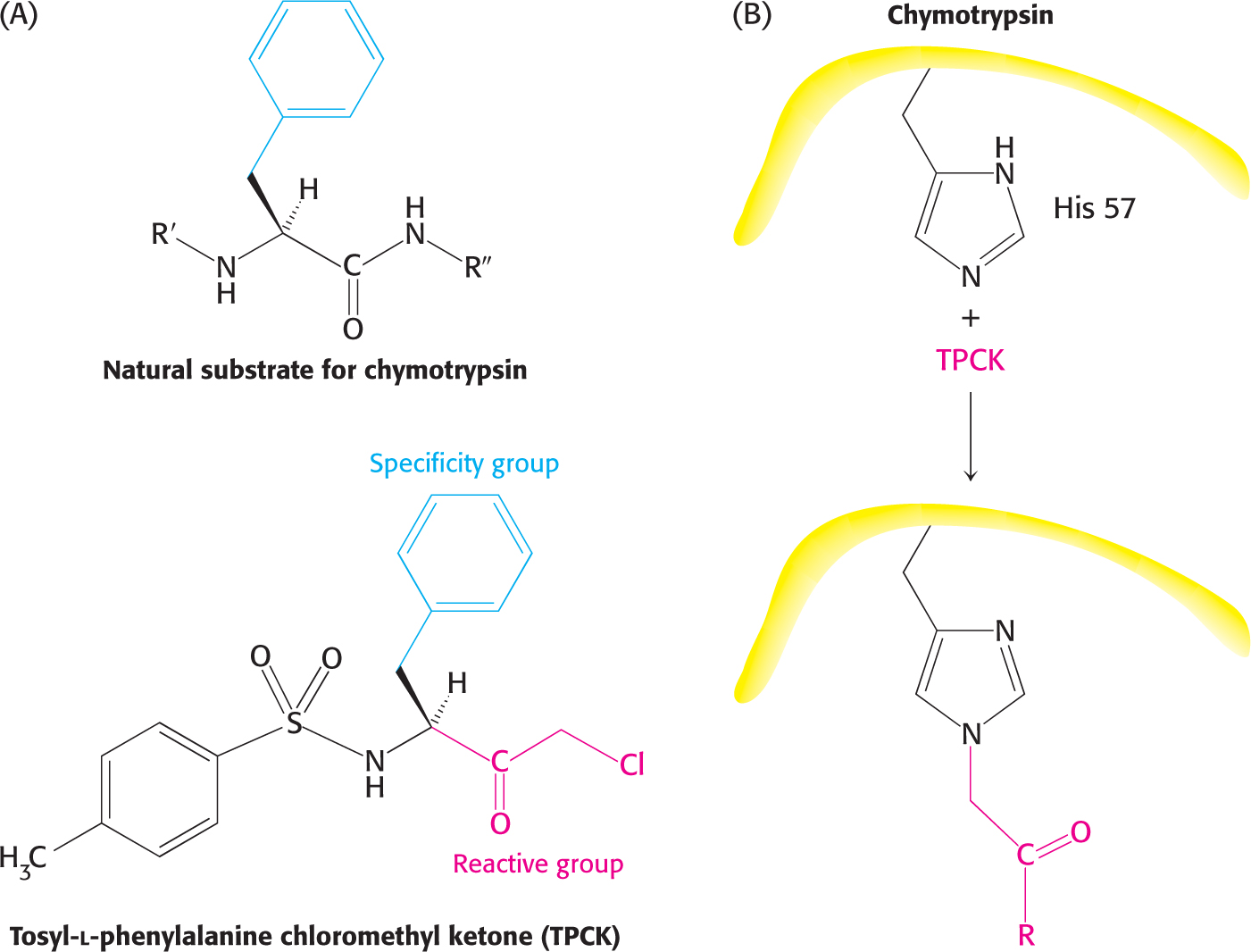
Affinity labels, also called substrate analogs, are molecules that covalently modify active-
Suicide inhibitors, or mechanism-
Transition-
 CLINICAL INSIGHT
CLINICAL INSIGHTPenicillin Irreversibly Inactivates a Key Enzyme in Bacterial Cell-Wall Synthesis
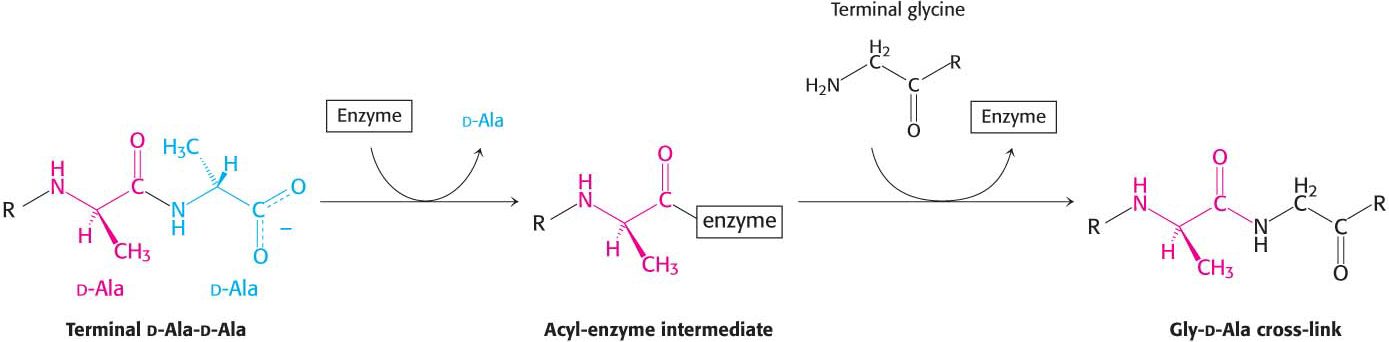
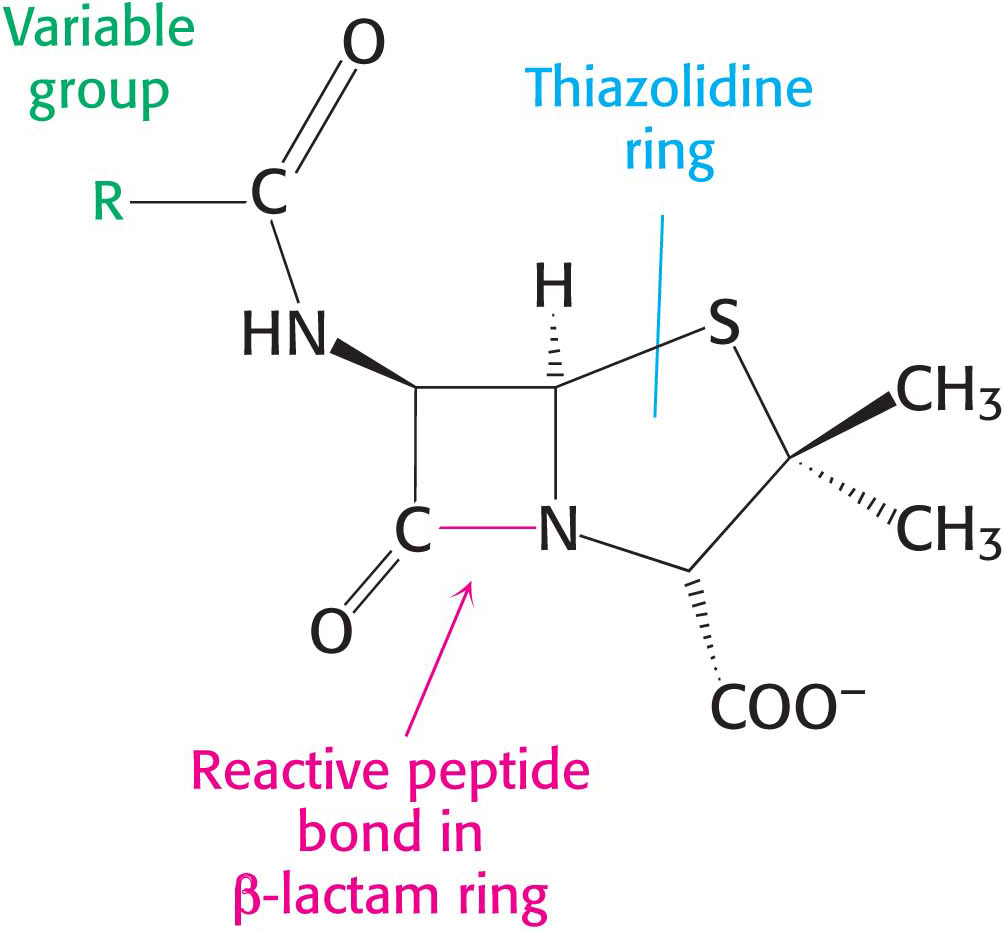
Penicillin, the first antibiotic discovered, consists of a thiazolidine ring fused to a β-lactam ring to which a variable R group is attached by a peptide bond (Figure 8.15). This structure can undergo a variety of rearrangements, and, in particular, the β-lactam ring is very unstable. Indeed, this instability is closely tied to the antibiotic action of penicillin, as will be evident shortly.
How does penicillin inhibit bacterial growth? Let us consider the bacterium Staphylococcus aureus, the most common cause of staph infections. Penicillin works by interfering with the synthesis of the S. aureus cell walls. The S. aureus cell wall is made up of a macromolecule, called a peptidoglycan (Figure 8.16), which consists of linear polysaccharide chains that are cross-
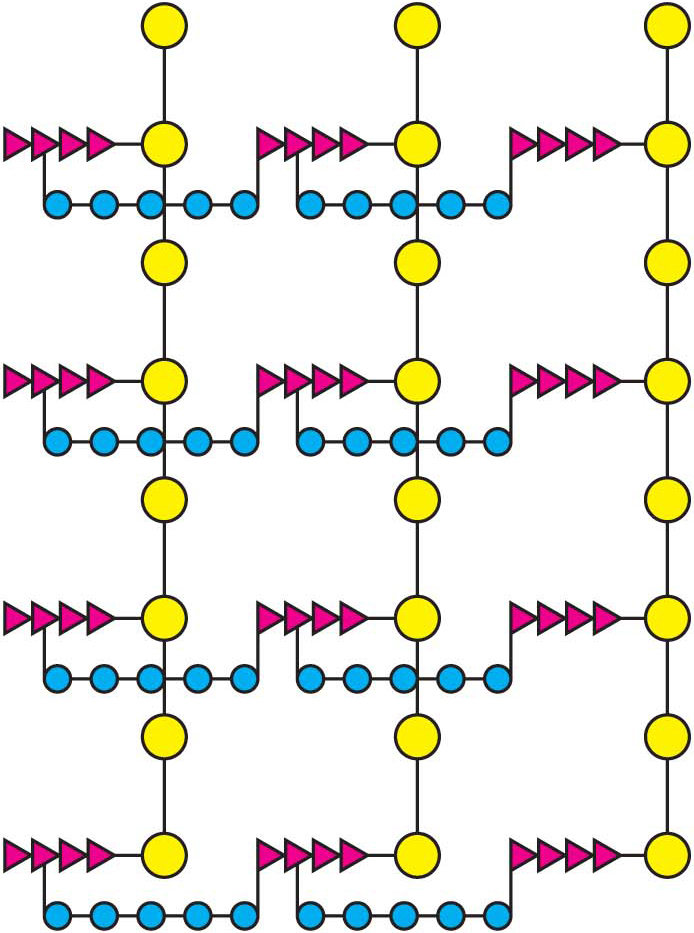
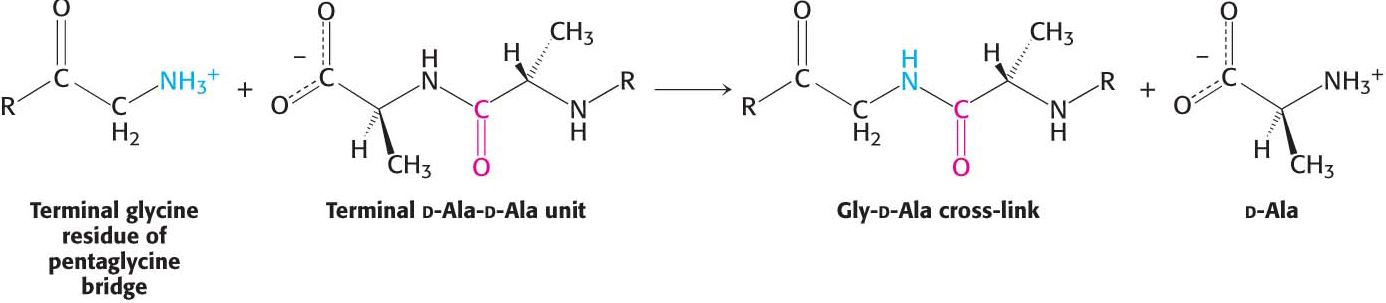
Penicillin inhibits the cross-