PROBLEMS
Question 8.1
1. Strategic solutions. What are the four basic catalytic strategies used by many enzymes?
Question 8.2
2. Keeping busy. Many isolated enzymes, if incubated at 37°C, will be denatured. However, if the enzymes are incubated at 37°C in the presence of substrate, the enzymes are catalytically active. Explain this apparent paradox.
Question 8.3
3. Controlled paralysis. Succinylcholine is a fast-
(a) As a safety measure, serum cholinesterase is measured before the examination takes place. Explain why this measurement is good idea.
(b) What would happen to the patient if the serum cholinesterase activity were only 10 units of activity per liter rather than the normal activity of about 80 units?
(c) Some patients have a mutant form of the serum cholinesterase that displays a KM of 10 mM, rather than the normal 1.4 mM. What will be the effect of this mutation on the patient?
Question 8.4
4. Specific action. How can group-
Question 8.5
5. Like bread and butter. Match the term with the description or compound. ✓ 6
Competitive inhibition Competitive inhibition Competitive inhibition Uncompetitive inhibition Uncompetitive inhibition Uncompetitive inhibition Noncompetitive inhibition Noncompetitive inhibition Noncompetitive inhibition | Sulfanilamide Doxycycline Inhibitor binds at the active site Binds to the enzyme– Lowers Vmax and  Roundup Inhibitor and substrate can bind simultaneously KM remains unchanged but Vmax is lower Vmax remains the same but the  increases increases |
Question 8.6
6. Mode of inhibition. The kinetics of an enzyme are measured as a function of substrate concentration in the presence and in the absence of 2 μM inhibitor. ✓ 6
|
|
Velocity (μmol minute−1) |
|
|---|---|---|
|
[S] (μM) |
No inhibitor |
Inhibitor |
|
3 |
10.4 |
4.1 |
|
5 |
14.5 |
6.4 |
|
10 |
22.5 |
11.3 |
|
30 |
33.8 |
22.6 |
|
90 |
40.5 |
33.8 |
(a) What are the values of Vmax and KM in the absence of inhibitor? In its presence?
(b) What type of inhibition is it?
Question 8.7
7. A different mode. The kinetics of the enzyme considered in problem 6 are measured in the presence of a different inhibitor. The concentration of this inhibitor is 100 μM. ✓ 6
|
|
Velocity (μmol minute−1) |
|
|---|---|---|
|
[S] (μM) |
No inhibitor |
Inhibitor |
|
3 |
10.4 |
2.1 |
|
5 |
14.5 |
2.9 |
|
10 |
22.5 |
4.5 |
|
30 |
33.8 |
6.8 |
|
90 |
40.5 |
8.1 |
(a) What are the values of Vmax and KM in the presence of this inhibitor? Compare them with those obtained in problem 6.
(b) What type of inhibition is it?
Question 8.8
8. Informative inhibition. What are the four key types of irreversible inhibitors that can be used to study enzyme function? ✓ 6
Question 8.9
9. Self-
Question 8.10
10. One for all and all for one. What is the catalytic triad, and what are the roles of the individual components in chymotrypsin activity?
Question 8.11
11. A burrow for oxyanions? What is the purpose of the oxyanion hole in chymotrypsin?
Question 8.12
12. Burst. What caused a “burst” of activity followed by a steady-
Question 8.13
13. Say no to cannibalism. If chymotrypsin is such an effective protease, why doesn’t it digest itself?
Chapter Integration Problems
Question 8.14
14. Mental experiment. Picture in your mind the velocity-
|
Experimental condition |
Vmax |
KM |
|---|---|---|
|
(a) Twice as much enzyme is used. (b) Half as much enzyme is used. (c) A competitive inhibitor is present. (d) An uncompetitive inhibitor is present. (e) A pure noncompetitive inhibitor is present. |
|
Question 8.15
15. Type 1 and type 2. In Section 7.2, we examined two types of bisubstrate reactions. Which of the two types best describes the action of chymotrypsin? Explain.
Challenge Problems
Question 8.16
16. Titration experiment. The effect of pH on the activity of an enzyme was examined. At its active site, the enzyme has an ionizable group that must be negatively charged in order for substrate binding and catalysis to take place. The ionizable group has a pKa of 6.0. The substrate is positively charged throughout the pH range of the experiment. ✓ 6
(a) Draw the V0-versus-
(b) Draw the V0-versus-
(c) At which pH will the velocity equal one-
Question 8.17
17. Mutant. Predict the effect of mutating the aspartic acid at the active site of chymotrypsin to asparagine.
Question 8.18
18. No burst. Examination of the cleavage of the amide substrate A by chymotrypsin very early in the reaction reveals no burst. The reaction is monitored by noting the color produced by the release of the amino part of the substrate (highlighted in orange). Why is no burst observed?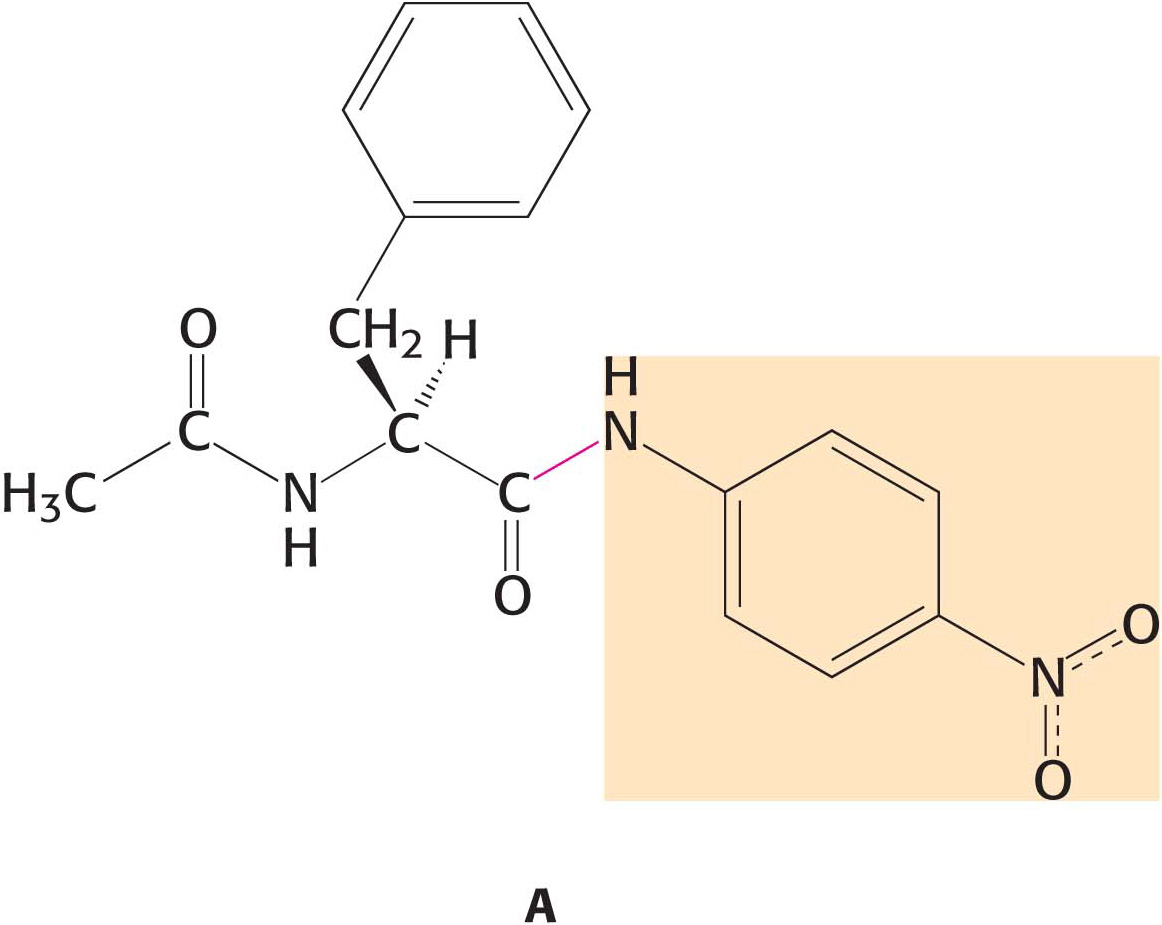
Question 8.19
19. Variations on a theme. Recall that TPCK, an affinity label, inactivates chymotrypsin by covalently binding to histidine 57. Trypsin is a protease very similar to chymotrypsin, except that it hydrolyzes peptide bonds on the carboxyl side of lysine or arginine. ✓ 6
(a) Name an affinity-
(b) How would you test the agent’s specificity?
Selected Readings for this chapter can be found online at www.whfreeman.com/