9.1 Hemoglobin Displays Cooperative Behavior
✓ 7 Explain how allosteric properties contribute to hemoglobin function.
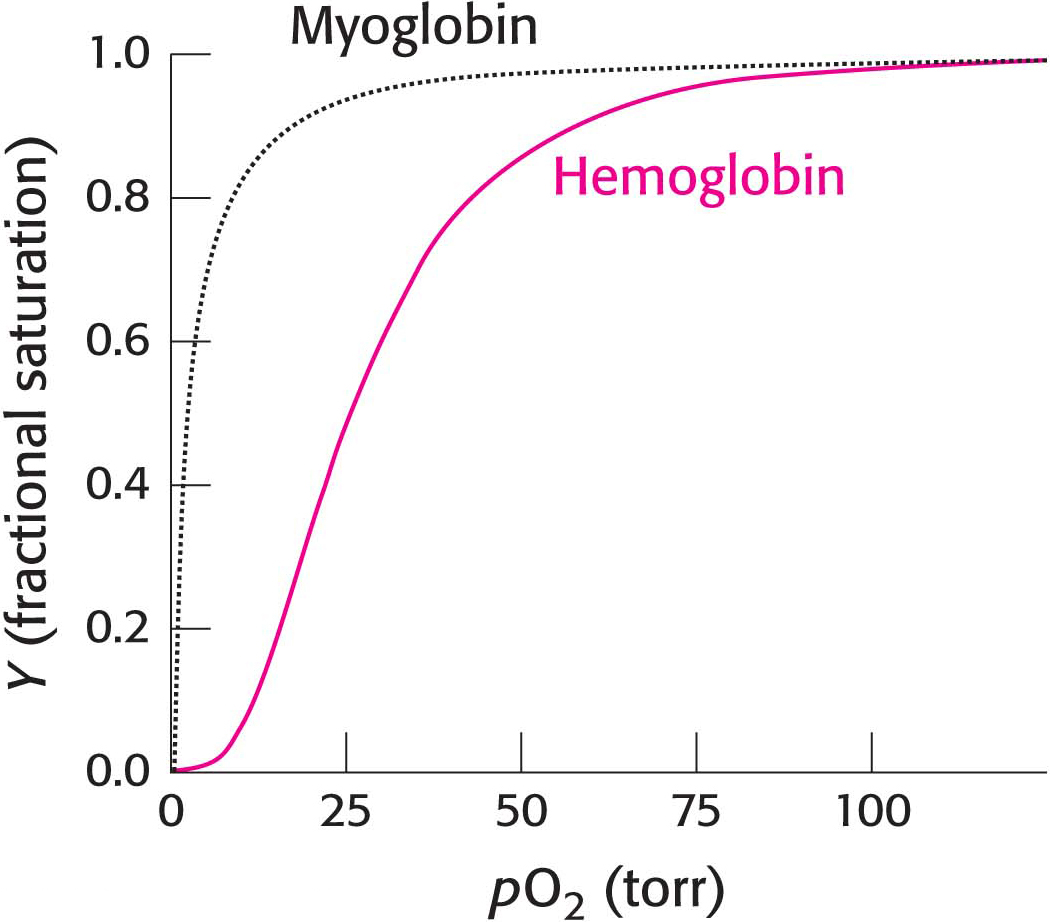
The binding of oxygen to hemoglobin isolated from red blood cells displays marked sigmoidal behavior, characteristic of cooperation between subunits, whereas myoglobin shows a hyperbolic curve (Figure 9.1). Indeed, the physiological significance of the cooperative effect in allosteric proteins is especially evident in the action of hemoglobin. Oxygen must be transported in the blood from the lungs, where the partial pressure of oxygen (pO2) is high (approximately 100 torr), to the tissues, where the partial pressure of oxygen is much lower (typically 20 torr).
DID YOU KNOW?
Partial pressure is the fraction of the total pressure of a mixture of gases that is due to one component of the mixture—in this case, oxygen. For instance, 1 atmosphere of pressure = 760 torr, or the pressure of a column of mercury 760 mm high. Air is 21% oxygen in dry air at sea level, and so the partial pressure of oxygen is 159 torr. In the alveoli of the lungs, the partial pressure of oxygen is 100 torr owing to the addition of water vapor and to the gas exchange that takes place.
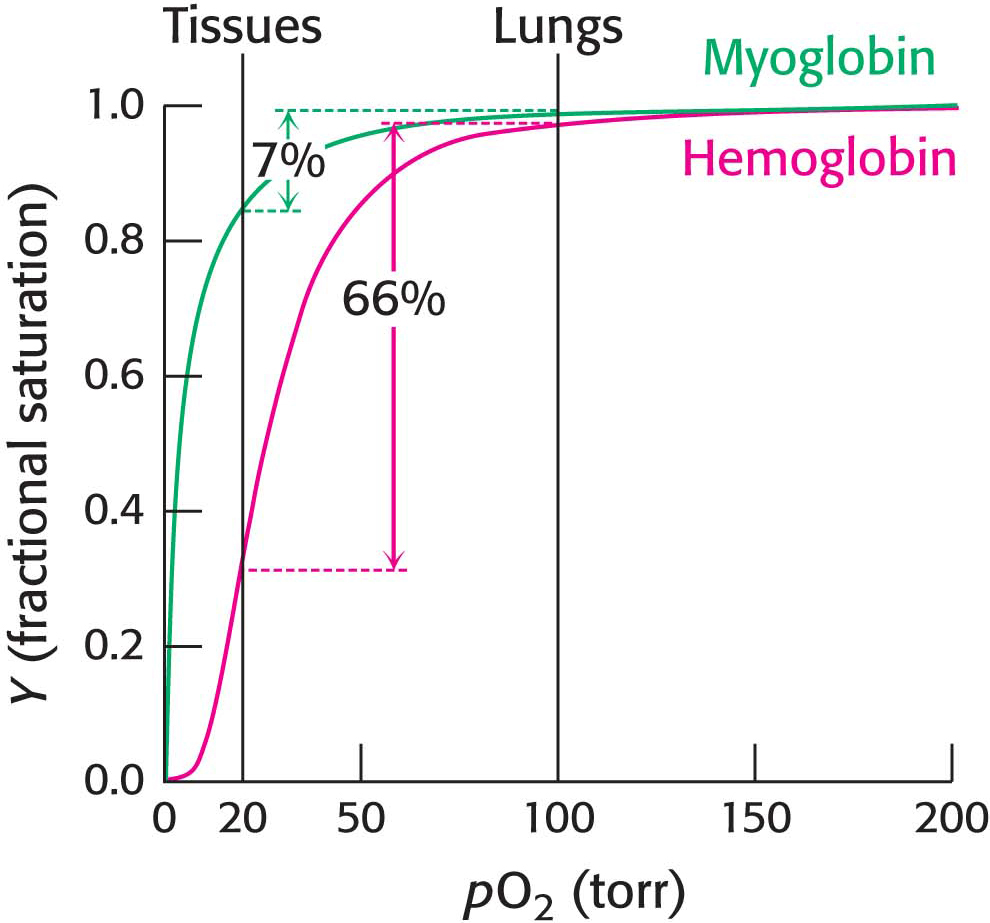
Let us consider how the cooperative behavior represented leads to efficient oxygen transport. In the lungs, hemoglobin becomes nearly saturated with oxygen, such that 98% of the oxygen-binding sites are occupied. When hemoglobin moves to the tissues, the oxygen saturation level drops to 32%. Thus, 66% (98 −32 = 66) of the potential oxygen-binding sites release oxygen in the tissues. If myoglobin, with its high affinity for oxygen, were a transport protein for oxygen, it would release a mere 7% of its oxygen under these conditions (Figure 9.2). Why does hemoglobin release only part of the oxygen that it carries? As you might guess from the discussion of allosteric enzymes (Section 7.3), allosteric regulators released at the tissues can further enhance oxygen release. We will examine these regulators later in the chapter.