9.3 Hemoglobin Binds Oxygen Cooperatively
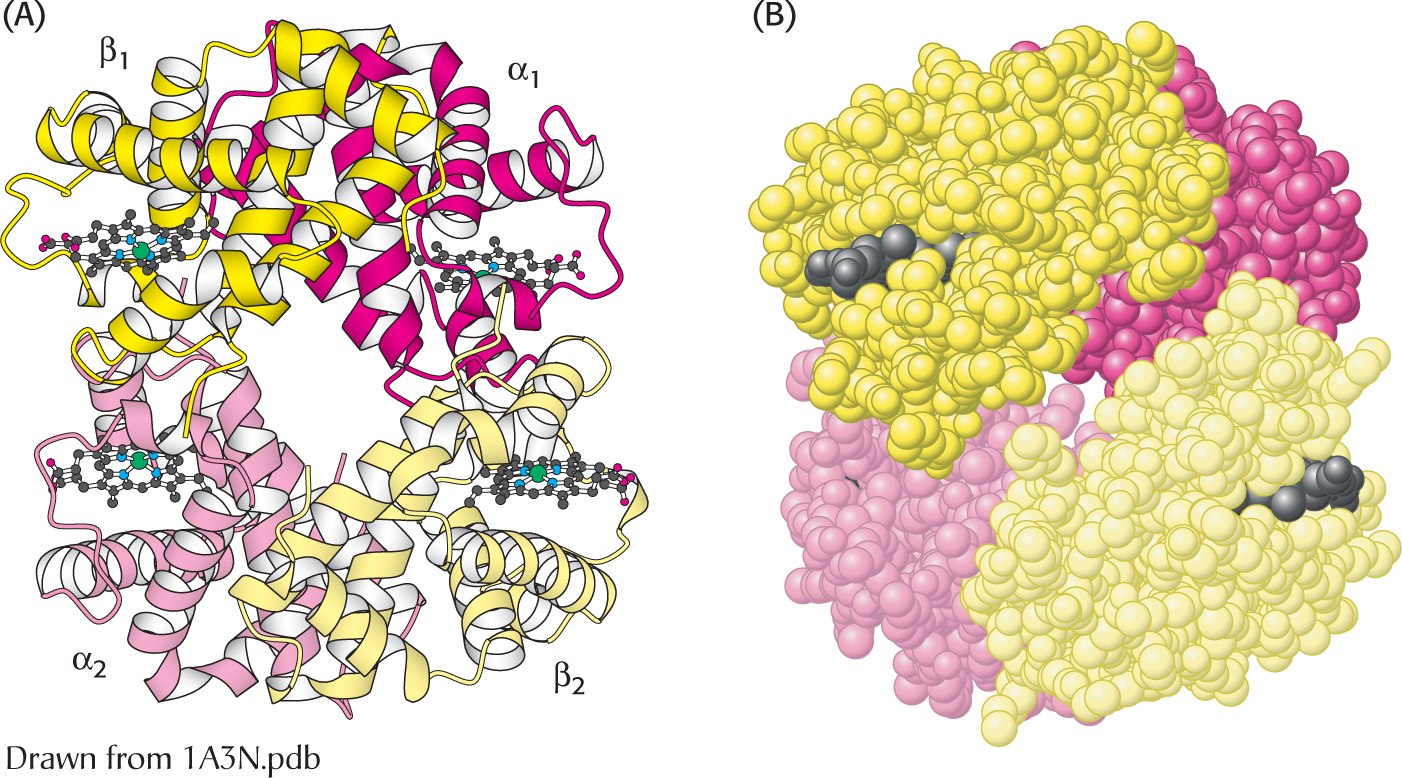
Like all allosteric proteins, hemoglobin displays quaternary structure. Human hemoglobin A, present in adults, consists of four subunits: two α subunits and two β subunits. The α and β subunits have similar three-dimensional structures and are similar to that of myoglobin. The three-dimensional structure of hemoglobin (Figure 9.6) is best described as a pair of identical αβ dimers (α1β1 and α2β2) that associate to form the hemoglobin tetramer. In deoxyhemoglobin, these αβ dimers are linked by an extensive interface, which includes, among other regions, the carboxyl terminus of each chain. Deoxyhemoglobin corresponds to the T state of hemoglobin, whereas oxyhemoglobin corresponds to the R state. Recallfrom the discussion of allosteric enzymes in Chapter 7 that the T state is less biochemically active than the R state. In regard to hemoglobin, the T state has alower affinity for oxygen than does the R state.
Page 153
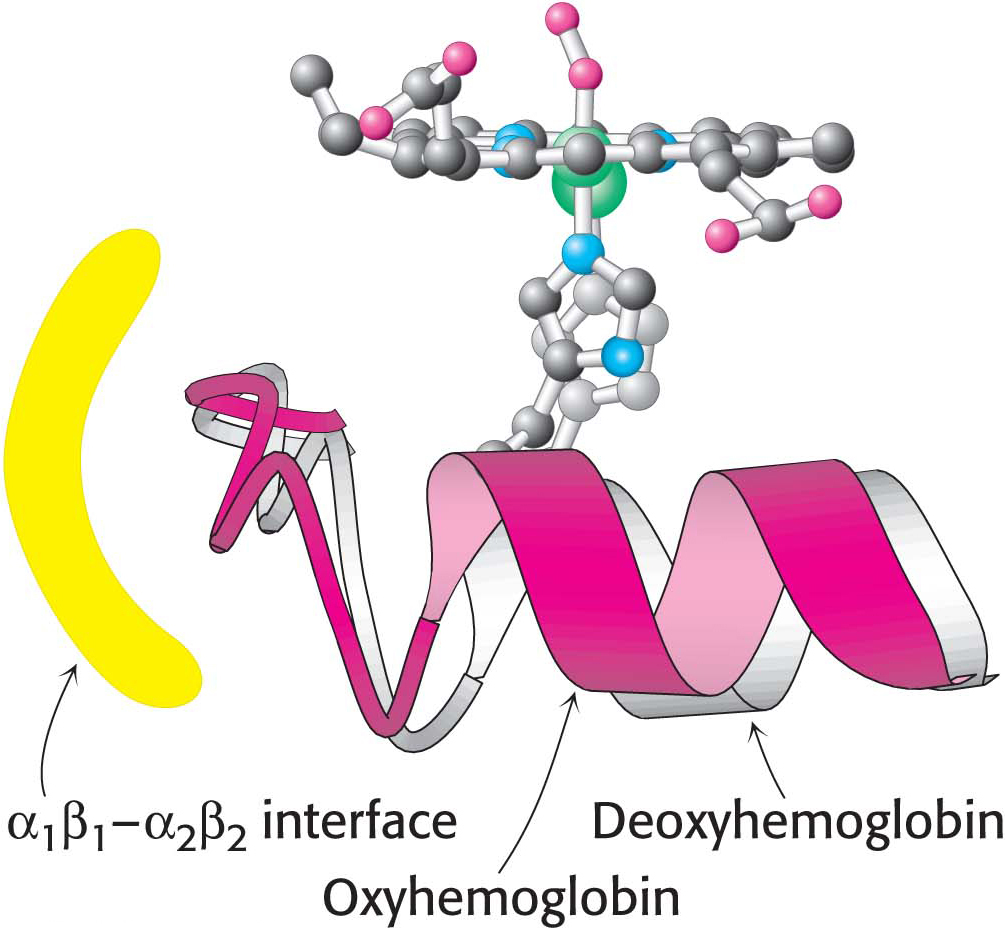
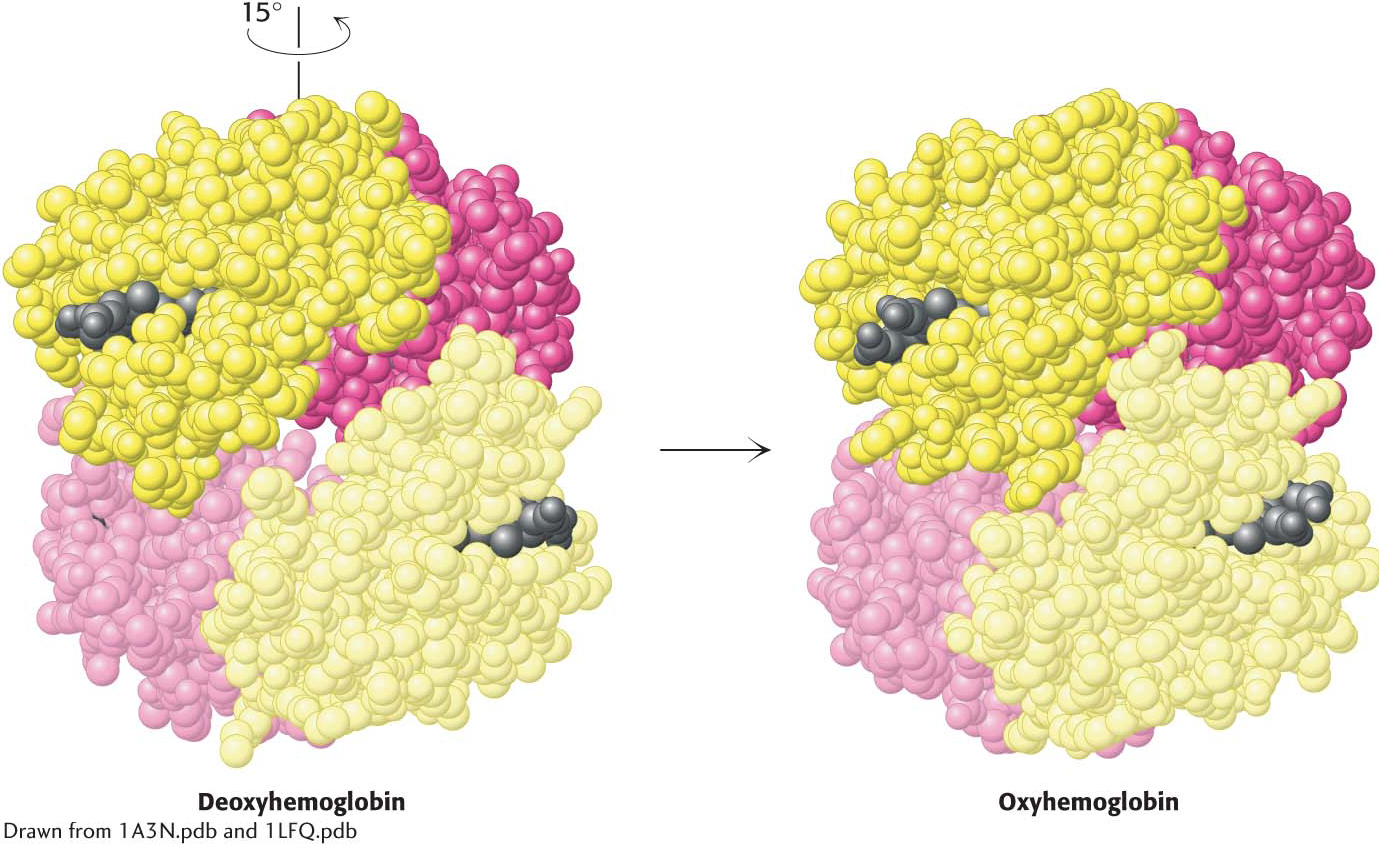
How does oxygen binding lead to the structural transition from the T state to the R state? When the iron ion moves into the plane of the porphyrin, the histidine residue bound in the fifth coordination site moves with it. This histidine residue is part of an α helix, which also moves (Figure 9.7). The carboxyl terminal end of this α helix lies in the interface between the two αβ dimers. Consequently, the structural transition at the iron ion is directly transmitted to the other subunits, resulting in substantial changes in quaternary structure that correspond to the T-to-R-state transition (Figure 9.8). The α1β1 and α2β2 dimers rotate approximately 15 degrees with respect to one another. The rearrangement of the dimer interface provides a pathway for communication between subunits: the presence of oxygen on one of the subunits is immediately communicated to the others so that the subunits change from T to R in concert.
Recall that we considered two models for cooperative binding (Section 7.3). Is the cooperative binding of oxygen by hemoglobin best described by the concerted or the sequential model? Neither model in its pure form fully accounts for the behavior of hemoglobin. Instead, a combined model is required. Hemoglobin behavior is concerted in that hemoglobin with three sites occupied by oxygen is almost always in the quaternary structure associated with the R state. The remaining open binding site has an affinity for oxygen more than 20-fold greater than that of fully deoxygenated hemoglobin binding its first oxygen atom. However, the behavior is not fully concerted, because hemoglobin with oxygen bound to only one of four sites remains primarily in the T-state quaternary structure. Yet this molecule binds oxygen three times as strongly as does fully deoxygenated hemoglobin, an observation consistent only with a sequential model. These results highlight the fact that the concerted and sequential models represent idealized cases, which real systems may approach but rarely attain.
Page 154