Chapter 5. EXPERIMENT FIVE (Part 1): Electrophilic Aromatic Substitution: Nitration of 4-Methylacetanilide
Introduction
Before lab, read about thin layer chromatography in Making the Connections (Ch3). As needed, review the electrophilic aromatic substitution reaction in your Organic Chemistry text.
Safety:
4-methylacetanilide: harmful if swallowed; causes skin irritation, serious eye irritation, and may cause respiratory irritation.
nitric acid: Causes severe skin burns and eye damage; may intensify fire; oxidizer.
ethanol: highly flammable liquid and vapor; toxic
ethyl acetate: highly flammable liquid and vapor; causes serious eye irritation; may cause drowsiness or dizziness
hexanes: highly flammable liquid and vapor; may be fatal if swallowed and enters airways; causes skin irritation; may cause drowsiness or dizziness; suspected of damaging fertility or the unborn child; may cause damage to organs (nervous system) through prolonged or repeated exposure if swallowed; toxic to aquatic life with long lasting effects
4-methyl- -nitroacetanilide: irritant

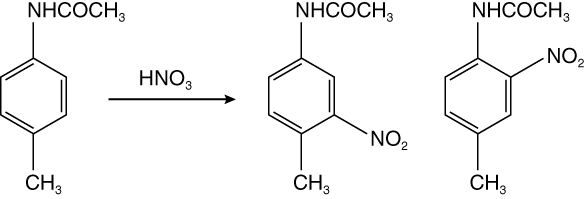
The electrophilic substitution of arenes is one of the most versatile ways of preparing functionalized aromatic compounds. A mixture of products can be obtained when there is more than a single substituent on the benzene ring. In this experiment, you will perform the nitration of 4-methylacetanilide, forming two mono-nitration products shown below:
The purity of the product can be ascertained by chromatography. Specifically, thin-layer chromatography (TLC) on silica gel plates provides a simple method for determining the purity of a product. In this experiment, you will characterize the crude product using TLC and melting point determination. These results will be compared to those after the crude product is purified by column chromatography in Part 2.

Experimental Procedure
Assemble an apparatus to afford removal of acid vapors during the course of the reaction by placing an inverted glass funnel over the mouth of a long neck round bottom flask and connecting the funnel to the aspirator (see set-up, illustrated below). Turn on the water to pull the fumes away from the reaction set-up.
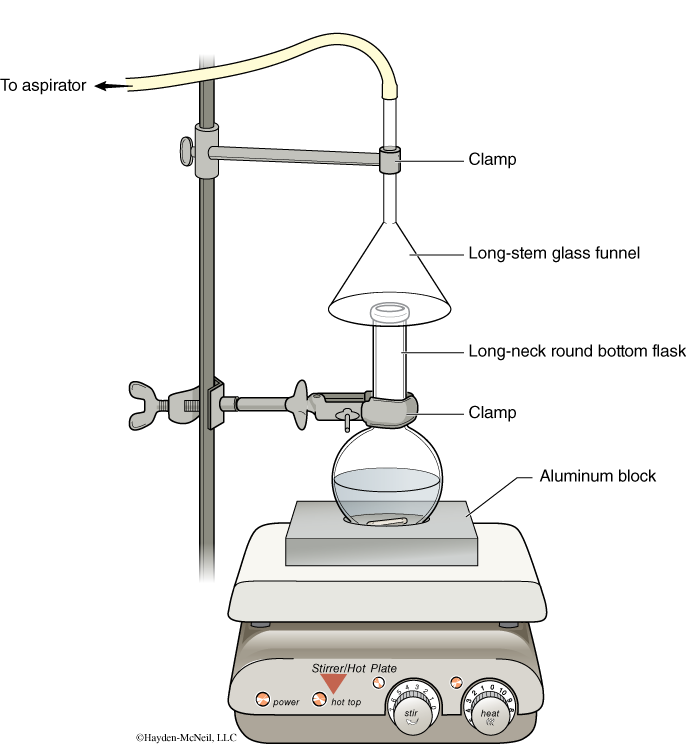
Place 2 mL of 70% nitric acid in a dry 5 mL long neck round bottom flask containing a stir bar. Add ~400 mg of 4-methylacetanilide to the flask, stirring well. Warm the solution with the hotplate set at 30-40ºC, and heat for about 15 min until the reaction turns dark orange (if the reaction turns brown it was overheated). Pour the resulting orange mixture into 10 mL of ice water. Filter the yellow product quickly by suction filtration with a Hirsch funnel. Wash the product with five 2-mL portions of ice-cold water. Allow air to be pulled through the solid for at least 5 minutes to dry. Save your crude product for Part 2!
TLC analysis: Your TA will dissolve a small amount of 4-methylacetanilide in 0.5-1.0 mL of ethyl acetate to be used as the reference during TLC analysis. Prepare a similar ethyl acetate solution of a small amount of crude product. Using an eppendorf tip, transfer a small amount of each liquid to a silica-gel TLC plate, according to the directions in Making the Connections. Record your sample starting point using a pencil (the ink from a pen may travel up the plate!). Using a 1:1 hexanes:ethyl acetate solution in a chamber made from a beaker and watch glass, develop the spotted TLC plate. You only need a small amount of 1:1 hexanes:ethyl acetate, just enough to cover the bottom of the beaker! Mark the final position of the solvent front as soon as you take the plate out of the developing chamber, using a pencil. Visualize the spots under UV light (your TA will demonstrate this), and calculate the RBfB values for each of the substances. Sketch your TLC plate in your laboratory notebook with recorded measurements.
WASTE DISPOSAL: All organic waste products must be discarded into the labeled liquid waste container. Nitric acid waste must be discarded in a separate container labeled “HNO3 waste only.”
Write-up Questions Part 1
What is your major product? What would you expect to be the major product of nitration of 2-methylacetanilide? Explain your reasoning.
You are asked to rapidly assay the purity of a sample, but do not have access to instrumentation. You decide to use TLC and melting point to check its purity. With 10:1 hexanes:ethyl acetate solvent, the sample gives one spot by TLC (Rf = 0.15) and has a melting point range of 115 – 125 °C. (a) Do you think the sample is pure? Why or why not? (b) Using the tools you have at hand (a melting point apparatus and TLC setup), what other data could you collect to test your hypothesis from part a? Give two possibilities.
Lab Report
The lab report for Experiment VIII (Parts 1 and 2) is combined: see schedule for due date. Prepare separate pre AND post lab notebook pages for the two weeks of this experiment.
Do not procrastinate! Start writing the lab report after Part I so you have ample time to ask your TA questions during office hours.