Chapter 1. Introduction
Undergraduate Laboratories Safety Rules
READ CAREFULLY
In order to avoid personal injuries and injuries to fellow students while performing experiments in your chemistry laboratory courses, you are required to follow the safety rules given below. Any questions about safety rules should be directed to your TA, instructor, Laboratory Supervisor, or Laboratory Director.
I. PERSONAL PROTECTIVE EQUIPMENT (PPE)
EYE PROTECTION
Approved safety goggles must be worn continuously while you are in the laboratory. Your regular glasses or contact lenses can be worn in the lab, but you MUST wear safety goggles over them.
All students will be issued a pair of safety goggles in the first lab course they take at UNC. If a student loses or breaks these safety goggles at any time during his/her tenure at UNC, the student must buy a new pair of safety goggles from the chemistry storeroom. If a student purchases safety goggles from an outside vendor, the Laboratory Supervisor must approve the safety goggles for use in the lab.
SKIN PROTECTION
Students, teaching assistants, and other staff members are to be appropriately clothed in the laboratory at all times, including check-in and checkout. Appropriate attire includes:
- A chemistry department approved lab coat. All students will be issued a lab coat during check in.
- Clothing that protects the individual's body from the neck to the ankles. Sleeveless shirts, tank tops, or other clothing that do not cover the shoulders, back, or abdominal area are not acceptable clothing to be worn in the teaching laboratory. Pants or skirts that do not cover the individual's ankles are not acceptable clothing to be worn in the teaching laboratory. Leggings are not considered pants and are therefore not allowed in lab. Absolutely no shorts are permitted in the lab.
- Footwear that covers the entire foot must be worn in the lab. Open-toed, open-heeled, and/or high heeled shoes, including sandals, flip flops, mules, clogs, pumps, moccasins, ballerina slippers, etc, are not acceptable footwear to be worn in the teaching laboratory.
- Gloves must be worn at all times while handling glassware and/or chemicals. Gloves must be removed before leaving the lab. If gloves become damaged, obtain a new pair. If gloves are heavily contaminated, remove, place in the hazardous waste, and obtain a new pair. Do not reuse gloves.
- Long hair must be tied back and loose clothing must be securely constrained under the lab coat when working in the laboratory.
II. WASTE DISPOSAL
- A paragraph at the end of each experimental section outlines “WASTE DISPOSAL” for the materials used in each lab procedure: READ AND FOLLOW THESE PROCEDURES CAREFULLY. Check with your TA if you have any questions.
- Chemical waste must be disposed of properly. Because of toxicity and flammability hazards, do not dispose of solvents by pouring them into the sink. Municipal sewage treatment plants are not equipped to remove these materials from sewage. Furthermore, with volatile and flammable materials, a spark or an open flame can cause an explosion in the sink or further down the drain. Solvents and some other liquids are disposed of in the hazardous waste plastic bottles.
- Never return unused reagents to stock bottles. Use the minimum amount of reagents to avoid waste disposal issues.
- Place solids in the plastic waste buckets.
- Keep all waste containers closed.
III. CONDUCT
- You are not permitted to enter the lab without TA supervision. And under no conditions are unauthorized or unsupervised experiments to be performed.
- Working alone in the lab is not permitted. You may work in the labs during specified hours and with proper supervision. You may not work in undergraduate labs without an instructor or teaching assistant present.
- Report any accidents, injuries, or hazardous spills, no matter how minor, to your TA or instructor at once. Incident report forms are inside the First Aid kits (by the main sink).
- Eating and drinking are prohibited in all lab spaces (this includes instrument rooms).
- Cell phones and all other electronic devices must be turned off and stored in your book bag.
- Always use hood ventilation when handling chemicals.
- Always transport chemicals in closed containers. Use secondary containment when leaving your lab room and going to an instrument room.
- Always Add Acids to water; never water to acids.
- Never aim the opening of a test tube, separatory funnel, or flask at yourself or at anyone else.
- Never leave anything unattended while it is being heated or is reacting rapidly.
- Always lubricate glass tubing and thermometers before inserting them into a stopper. Always wrap toweling around them while inserting. Hold tubing and thermometers close to the point of insertion.
- No open flames in the lab.
- Never pipet by mouth--use a pipet bulb.
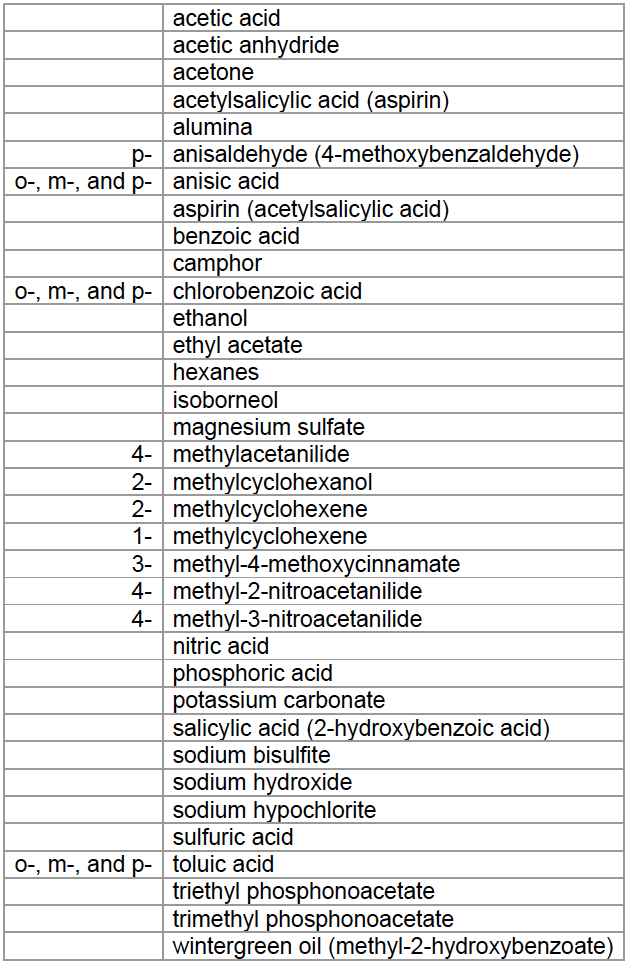
IMPORTANT: Chemical Reagents Used In CHEM262L
If you are currently pregnant or become pregnant during the semester of this laboratory course, you are encouraged to consult with your personal physician, or contact the Student Health Services to discuss the advisability of continuing this course.
HONOR CODE and ACADEMIC INTEGRITY
The Department of Chemistry faculty adopted the following policy on September 9, 1977.
“Since all graded work (including homework to be collected, quizzes, papers, mid-term examinations, final examination, research proposals laboratory results and reports) may be used in the determination of academic progress, no collaboration on this work is permitted unless the instructor explicitly indicates that some specific degree of collaboration is allowed. This statement is not intended to discourage students from studying together or working together on assignments which are not to be collected.”
The guiding principle of academic integrity is that the work submitted by a student must be that student’s own work. In this course students will sometimes be required to work in pairs or groups to collect experimental data. This can lead to misunderstandings regarding academic integrity. In those cases when you work with other students, you must clearly indicate on your Title Page who your partner or partners were.
When writing up your lab report there is no collaborative work. You must write your own report, answer your own questions, and work up your own data. If you are having difficulties or have questions you need to see your TA for help. Collaboration on lab reports is a violation of the University Honor Code and will be treated as such.
A second area where misunderstandings of academic integrity arise is with regards to when you should reference external sources in your lab report. The submission of any material that is substantially the same as some other written document or source (i.e., a journal article, a textbook, a lab manual, a book) that is not properly referenced constitutes a violation of academic integrity. Using someone else’s words or ideas without giving credit for their work is called plagiarism. Furthermore, simply rearranging the words from a source to make them seem like your own words is also plagiarism.
The following situations below will be treated as honor code violations.
- Unauthorized collaboration. NOTE: Unauthorized collaboration is defined differently for each lab course. Please read carefully. All lab reports must be written independently.
- Plagiarism. The ideas presented in your report must be your own. If you present someone else’s ideas or work (from books, old lab reports, the Web, the lab manual) as your own, this is plagiarism. You can present facts from an outside source, as long as you properly reference the source.
- Allowing students to use your work as their own. Do not allow your partner or other students to have access to your lab reports. You may share data if you collected the data together, but everything else (calculations, graphs, tables) must be done alone.
- Using old lab reports, even if you just want to glance over them, is an honor code violation.
- Do not rearrange a paragraph or some other piece of work that is not yours in the hope of disguising the work as your own.
- Using an old lab manual from a previous semester.
Established by the Undergraduate Labs Committee, April 2014
COURSE OBJECTIVES
In the organic chemistry lab you will acquire hands-on experience with many different techniques associated with manipulating organic compounds. Chemistry 262L affords the opportunity to perform the reactions that you have learned about in organic chemistry lecture courses.
column chromatography
crystallization
distillation
extraction
gravity filtration
reflux
sublimation
suction filtration
thin-layer chromatography
In learning the above laboratory techniques, you will perform the following organic reactions:
Dehydration of alcohols (the E1/E2 reaction)
Nitration of an aromatic compound (Electrophilic Aromatic Substitution)
Oxidation of a secondary alcohol to a ketone
Hydrolysis of an ester
Esterification of an alcohol
The Wittig reaction
And you will learn how Green Chemistry can be incorporated into organic syntheses.
You will utilize NMR, GC, UV-Vis, and/or melting points to evaluate your products.
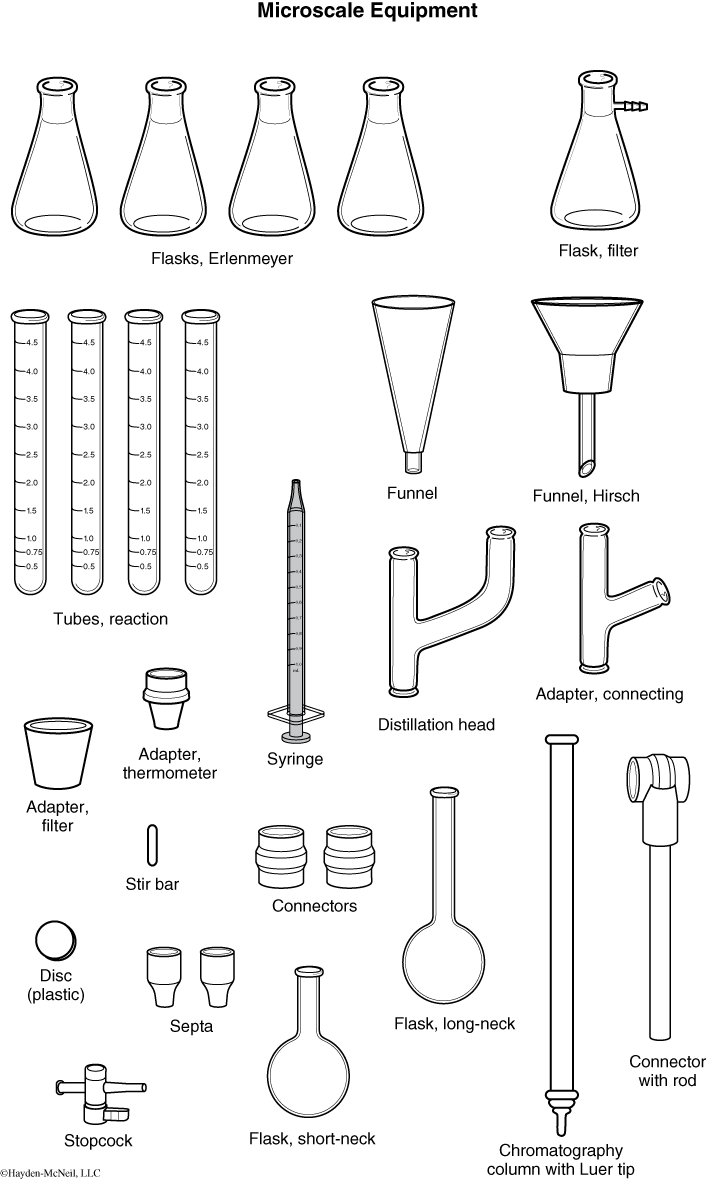
MICROSCALE REACTIONS
Many of the experiments in Chemistry 262L are conducted using “microscale” equipment. By working on a reduced scale, smaller quantities of chemicals are used, leading to the following benefits:
- Reduction in the time required to conduct a procedure, which in turn allows you to repeat failed experiments or to perform additional ones, thereby gaining experience and confidence in your laboratory techniques
- Substantial reduction of safety hazards, both in terms of possible fire and explosions, and of exposure to toxic substances
- Minimization of waste generation and waste disposal costs
- Lower cost of chemicals and glassware replacement in case of breakage
In conducting the experiments in this course, you will use experimental setups that may differ from any that you may have used before in a chemistry laboratory. Some equipment is illustrated below.
Also, in measuring quantities of materials on a small scale, be aware of the following:
- If the instructions call for > 1 mL, this can be conveniently measured with your graduated cylinder. You do not need to spend a lot of time measuring solvents precisely.
- If the experimental instructions call for < 1 mL and/or measurements to one or two decimal places, you can use your syringe to measure this amount accurately. Another way to measure the amount of a liquid reagent accurately is to use the known density of the compound to calculate how many grams (or mg) you will be adding. Then, with a disposable pipet, add the compound dropwise to a small vial or test tube, which is placed on the balance. Additionally, you can convert mass to volume using the known density of the compound, if it is more convenient to measure the reagent by volume.
COURSE INFORMATION SUMMARY
- Please refer to the Sakai site for the course syllabus, additional information, and resources.
- Everyone is expected to observe safety rules to avoid hazards to yourself and others in the lab. Repeated failure to observe safety rules will result in removal from the lab.
- In order to be enrolled in CHEM262L, you must have completed, with a passing grade, CHEM261/261H, and have completed or be currently enrolled in CHEM262. You must have completed, with a passing grade, CHEM241L. If you were first enrolled prior to Fall 2009, please see the instructor.
- Your grade for this course will be based on pre-lab quizzes, your lab notebook (pre-lab and final), NMR assignments, lab reports, final exam, and daily evaluations by your TA.
- Read the make-up lab policy posted on Sakai. This may change each semester.
- It is a requirement of the course that you check out of your lab locker before the end of the semester. Failure to do so results in a $100 improper check out fee, and may include additional charges related to broken and/or missing glassware.
- NMR (nuclear magnetic resonance) is a very important analytical technique for characterizing organic compounds. You will complete several NMR assignments. If you have a weak background in NMR, it is important that you work early to get up to speed in this area. Review the chapter in your textbook and Ch 2 in Making Connections. An excellent website for NMR spectroscopy background and interpretation is: http://www.cem.msu.edu/~reusch/VirtualText/Spectrpy/nmr/nmr1.htm
Sample Calculations
Conversion of Masses and Volumes
Reactions occur between numbers of moles of reactants depending on the stoichiometric coefficients of these reactants. In the laboratory we measure quantities of materials as weights or volumes.
For a pure solid or liquid, moles can be calculated using the following equation:
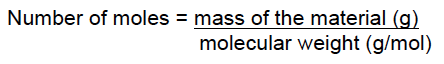
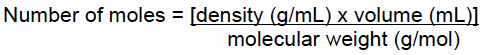
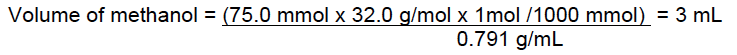
The amount of solute in a solution depends on the type of solution used. The most popular form is moles/volume (molarity M), weight/weight (usually used for concentrated acids such as HCl, HNO3 and H2SO4), or weight/volume.
Number of moles of NaOH = 11.3 g / 40 g/mol = 0.3 mol
Yield Calculation
Yield can be expressed in different forms depending on the type of transformation.
This is used when no chemical reaction occurred such as in purification procedures (distillation, recrystallization or sublimation).

This is used when a chemical reaction occurred. In order to calculate the % yield you need to calculate the theoretical yield first. The theoretical yield is defined as the maximum amount of product that can be obtained by a reaction from a given amount of reactants. The maximum amount of product depends on the Limiting Reagent, which is the reactant that is entirely consumed when a reaction goes to completion.
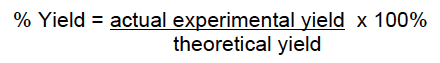
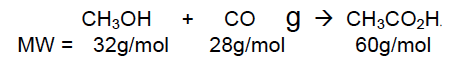
moles of CO = 10.0 g/ 28 g/mol = 0.36 mol
moles of CH3OH = 15.0 g/ 32 g/mol = 0.47 mol
Therefore, CO is the limiting reagent.