Geobiological Events in Earth’s History
The geologic time scale divides time based on the comings and goings of fossil assemblages (see Chapter 8). These biological patterns provide a convenient ruler for subdividing Earth’s history, but they were almost always associated with global environmental changes. At many of the major boundaries in the geologic time scale, Earth experienced a one-time event that caused dramatic changes in conditions for life. Some of these changes were triggered by organisms themselves, others by geologic events, and still others by forces from outside the Earth system.
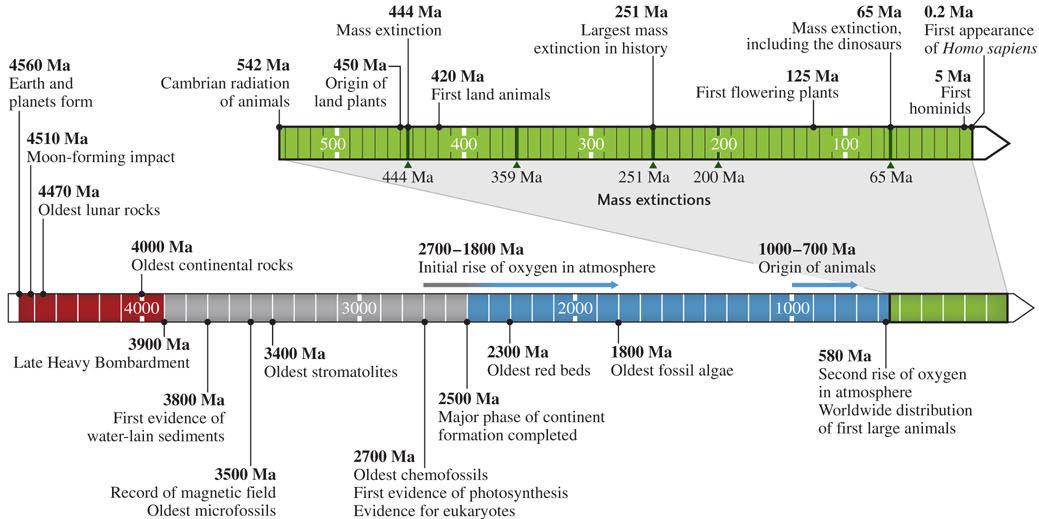
We will now study a few of these dramatic events in Earth’s history—events in which the link between life and the physical environment is clearly visible. Figure 11.12 shows the great antiquity of life on Earth and the timing of several of these major events.
Origin of Life and the Oldest Fossils
When Earth first formed some 4.5 billion years ago, it was lifeless and inhospitable. A billion years later, it was teeming with microorganisms. How did life begin? Along with other grand puzzles such as the origin of the universe, this question remains one of science’s greatest mysteries.
297
The question of how life may have originated is very different from the question of why life originated. Science offers an approach only to understanding the “how” part of this mystery because, as you may recall from Chapter 1, it uses observations and experiments to create testable hypotheses. These hypotheses may explain the series of steps involved in the origin and evolution of life, and they can be tested by searching for evidence in the fossil and geologic records. However, observations and experiments do not provide a testable approach to the question of why life evolved.
The fossil record tells us that single-celled microorganisms were the earliest forms of life, and that they evolved into all the multicellular organisms that are found in the younger parts of the geologic record. The fossil record also shows us that most of life’s history involved the evolution of microorganisms. We can find microfossils in rocks 3.5 billion years old, yet we can conclusively identify fossils of multicellular organisms only in rocks younger than 1 billion years. It therefore appears that microorganisms were the only organisms on Earth for at least 2.5 billion years!
The theory of evolution predicts that these first microorganisms—and all life that came after them—evolved from a universal ancestor (see Figure 11.5). What did this universal ancestor look like? We really don’t know, but most geobiologists agree that it must have had several important characteristics. The most crucial of these would be genetic information: instructions for growth and reproduction. Otherwise, it would have had no descendants. The universal ancestor must also have been composed of carbon-rich compounds. As we have seen, all organic substances, including organisms, are made principally of carbon.
How did the universal ancestor arise? One approach to answering this question would be to search for clues in rocks. However, well-preserved fossils are found only in sedimentary rocks that have not been significantly affected by metamorphism or deformation. There are no well-preserved sedimentary rocks from the time when life first evolved, so scientists must use other approaches. Laboratory chemists have played an important role here.
Prebiotic Soup: The Original Experiment on the Origin of Life
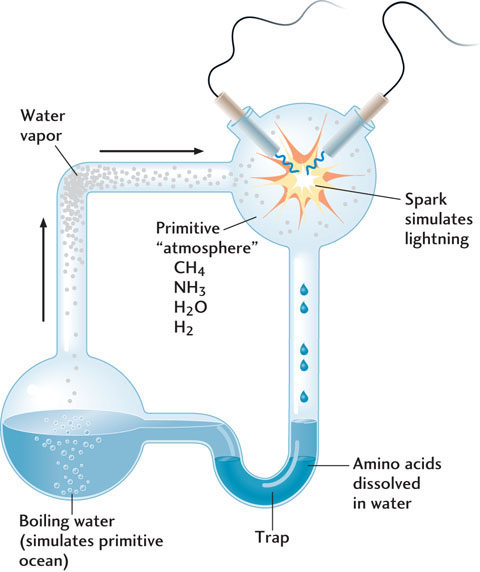
In laboratory experiments that probe the origin of life, scientists have tried to recreate some of the environmental conditions thought to have existed on Earth before life arose. In the early 1950s, Stanley Miller, a graduate student at the University of Chicago, did the first experiment designed to explore life-building chemical reactions on early Earth. His experiment was amazingly simple (Figure 11.13). At the bottom of a flask, he created an “ocean” of water that he then heated to create water vapor. The water vapor emitted from the ocean was mixed with other gases to create an “atmosphere” containing some of the compounds thought to be most abundant in Earth’s early atmosphere: methane (CH4), ammonia (NH3), hydrogen (H2), and the water vapor. Oxygen—an important gas in Earth’s atmosphere today—was probably absent at that time. In the next step, Miller exposed this atmosphere to electrical sparks (“lightning”), which caused the gases to react with one another and with the water in the ocean.
298
The results were impressive. The experiment yielded compounds called amino acids in addition to other carbon-bearing compounds. Amino acids are the fundamental building blocks of protein molecules, which are essential for life. Thus, if you want to build an organism, creating amino acids is a good place to start. Miller’s discovery was exciting because it showed that amino acids could have been abundant on early Earth. It led to the hypothesis that Earth’s ocean and atmosphere formed a sort of “prebiotic soup” of amino acids in which life originated. Other researchers have suggested that our universal ancestor contained genetic material that enabled amino acids to form proteins, which it then relied on for self-perpetuation.
The “prebiotic soup” hypothesis predicted that early planetary materials might contain amino acids. That prediction was borne out years later when, in 1969, a meteorite hit Earth near Murchison, Australia. When geologists analyzed it, they discovered that the Murchison meteorite contained many (about 20) of the amino acids that Miller had created in the laboratory! In fact, it even had similar relative amounts of those amino acids.
The message of all these discoveries is the same: amino acids could have formed on a planet without oxygen. But the opposite is also true: where oxygen is present, amino acids do not form, or are present only in tiny amounts. This is one of several reasons why Earth scientists think early Earth was a planet without oxygen.
The Oldest Fossils and Early Life
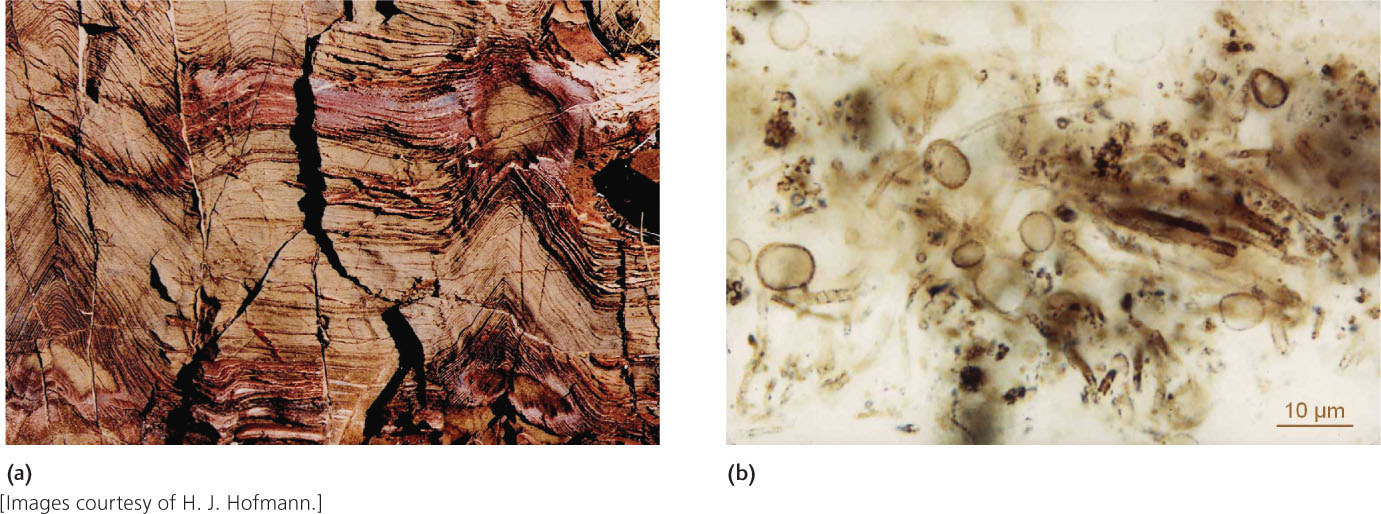
Whatever the processes by which life originated, the oldest potential fossils on Earth suggest that it had originated by 3.5 billion years ago. Stromatolites shaped like small cones provide some of the best available evidence for life at this time (Figure 11.14a). Stromatolites are common in continental cratons and have been identified in sedimentary rocks of early Archean age. In addition, the ratios of carbon isotopes found in some early Archean rocks show values that could have been produced only by biological processes (see the Practicing Geology Exercise at the end of the chapter). The oldest fossils that preserve possible morphological evidence for life are tiny threads that are similar in size and appearance to modern microorganisms, encased in chert. These features were found in formations in Western Australia that may be as old as 3.5 billion years, although their interpretation as microfossils remains controversial. Younger, better-preserved microfossils occur in the 3.2-billion-year-old Fig Tree formation of South Africa and in the 2.1-billion-year-old Gunflint formation of southern Canada (Figure 11.14b). The Gunflint fossils, discovered in 1954, were the first ever discovered in Precambrian rock, and they set off a tidal wave of research that continues to this day. In the past 50 years, we have seen, in many new localities, just how ancient life on Earth is and how well it can be preserved under the right geologic circumstances.
299
Most geobiologists agree that there was life on Earth 3.5 billion years ago, but are uncertain about how those early organisms functioned or obtained energy and nutrients. Some scientists argue that the oldest organisms on the universal tree of life were chemoautotrophic, obtaining their energy directly from chemicals in the environment. Furthermore, those oldest organisms may have been hyperthermophilic. This possibility suggests that life may have originated in very hot water, such as that in hot springs or hydrothermal vents on mid-ocean ridges, where sunlight was unavailable as an energy source, but chemicals were abundant (Figure 11.15).

Chemofossils and Eukaryotes
Form and size alone are not enough to allow us to deduce the function of microorganisms, so microfossils are ultimately limited in the information they can provide. Additional information can be gleaned from chemofossils, the chemical remains of organic compounds made by ancient microorganisms while they were alive. When an organism dies, most of the organic compounds in its body are quickly broken down into much smaller molecules, usually by heterotrophs. Some of these molecules, however, are very stable and resist recycling. Cholestane, for example, is a remarkably durable substance, made only by eukaryotes, that is very similar to the well-known compound cholesterol. Cholestane chemofossils have been identified in 2.7-billion-year-old rocks from Western Australia. The presence of these chemofossils tells us that single-celled eukaryotic microorganisms must have emerged by that time. It is the eukaryotes that would eventually evolve into multicellular organisms, including animals, but not until much later.
Origin of Earth’s Oxygenated Atmosphere
The rise of oxygen—the stuff we breathe—is another important milepost in the history of interactions between life and its environment. As we learned in Chapter 9, Earth’s early atmosphere contained little oxygen. Our current oxygen-rich atmosphere was produced by early life through photosynthesis. Remarkably, the same Australian rocks that preserve chemofossil evidence of eukaryotes also preserve chemofossil evidence of cyanobacteria. Because of this evidence, geologists believe that photosynthesis had become an important metabolic process by 2.7 billion years ago. Thus, one group of organisms (cyanobacteria) permanently altered Earth’s environment by changing the composition of its atmosphere, while another group of organisms (eukaryotes) was influenced by that change to evolve in new directions.
The oxygenation of Earth’s atmosphere probably occurred in two main steps, separated by more than a billion years. The first major increase began with the evolution of the cyanobacteria. The oxygen they produced reacted with iron dissolved in seawater, causing iron oxide minerals, such as magnetite and hematite, and silica-rich minerals, such as chert and iron silicates, to precipitate and sink to the seafloor. These minerals accumulated in thin, alternating layers of sediments called banded iron formations (Figure 11.16a). Iron is soluble in water when oxygen concentrations are low, as would have been the case on Earth before cyanobacteria evolved. When oxygen concentrations are high, however, iron reacts with oxygen to form highly insoluble compounds. Therefore, the oxygen produced by cyanobacteria would have immediately caused iron to precipitate from seawater and sink to the seafloor. This process would have continued until most of the dissolved iron was used up, allowing oxygen to accumulate in the ocean and atmosphere.

300
Atmospheric oxygen concentrations began to build about 2.4 billion years ago and reached an initial plateau about 2.1 billion to 1.8 billion years ago, when the first eukaryotic fossils, of a type of algae, entered the geologic record (Figure 11.16b). The large size of these organisms—at least 10 times larger than anything that came before them—is thought to be a consequence of the oxygen increase. This time also marks the first appearance of red beds, unusual stream deposits of sandstones and shales bound together by iron oxide cement, which gives them their red color (Figure 11.16c). The presence of iron oxides in these deposits indicates that oxygen must have been present in the atmosphere to precipitate them.
After eukaryotic algae came on the scene, not much happened for over a billion years. Then, about 580 million years ago, atmospheric oxygen concentrations rose dramatically, almost to their modern level. The reason for this second increase is still not understood, though it may be related to an increase in the burial of organic carbon by sedimentation. In a process somewhat similar to the one that produces banded iron formations, oxygen reacts easily with organic matter, usually with the help of microorganisms. Thus, as long as there is organic matter around, oxygen will be used up. If organic matter is removed from the system by burial in sediments, however, it cannot react with the available oxygen. Thus, the second step in the rise of atmospheric oxygen might have been related to an increase in sediment production. Such an increase may have occurred when mountains were built—and then eroded—during global tectonic events, such as the assembly of supercontinents. In any case, the consequences were dramatic: the first large multicellular animals suddenly appeared, and all the modern groups of animals evolved shortly thereafter, ushering in the Phanerozoic eon with its wonderfully complex and diverse organisms.