Chemical Weathering
Chemical weathering occurs when minerals react with air and water. In these chemical reactions, some minerals dissolve. Others combine with water and atmospheric components such as oxygen and carbon dioxide to form new minerals. We begin our investigation by examining the chemical weathering of feldspar, the most abundant mineral in Earth’s crust.
The Role of Water: Feldspar and Other Silicates
Feldspar is one of many silicates that are altered by chemical reactions to form clay minerals. Feldspar’s behavior during weathering helps us understand the weathering process in general, for two reasons:
- 1. Feldspar is a key mineral in a great many igneous, sedimentary, and metamorphic rocks and is one of the most abundant minerals in Earth’s crust.
- 2. The chemical processes that characterize feldspar weathering also characterize weathering in many other kinds of minerals.
Feldspar is one component of granite, which, as you will recall, is made up of several different minerals, all of which decay at different rates. A sample of unweathered granite is hard and solid because an interlocking network of feldspar, quartz, and other crystals holds it tightly together. When the feldspar is transformed by weathering into a loosely adhering clay, the network is weakened and the mineral grains are separated (Figure 16.2). In this instance, chemical weathering, by producing the clay, also promotes physical weathering because the rock now fragments easily along widening cracks at the boundaries between grains.
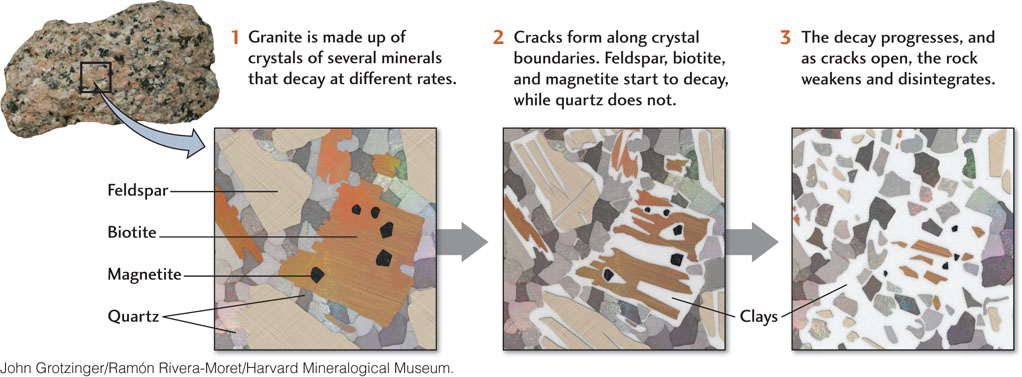
The white to cream-colored clay produced by the weathering of feldspar is called kaolinite, named for Gaoling, a hill in southwestern China where it was first obtained. Chinese artisans had used pure kaolinite as the raw material of pottery and porcelain for centuries before Europeans borrowed the idea in the eighteenth century.
Only in the arid climates of some deserts and polar regions does feldspar remain relatively unweathered. This observation points to water as an essential component of the chemical reaction by which feldspar becomes kaolinite. Kaolinite is a hydrous aluminum silicate. In the reaction that produces it, the solid feldspar undergoes hydrolysis (a decomposition reaction involving water; from hydro, “water,” and lysis, “to loosen”). The feldspar is broken down and also loses several chemical components, while kaolinite gains water.
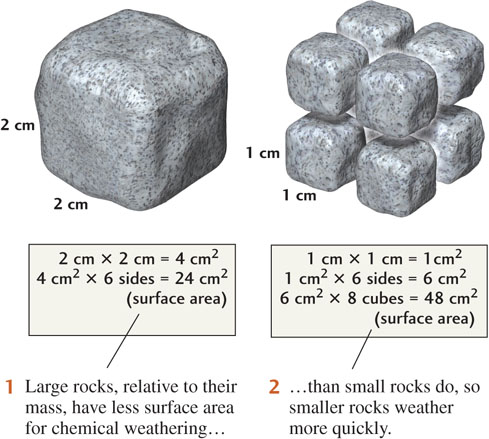
The only part of a solid that reacts with a fluid is the solid’s surface, so as we increase the surface area of the solid, we speed up the reaction. For example, as we grind coffee beans into finer and finer particles, we increase the ratio of their surface area to their volume. The finer the coffee beans are ground, the faster their reaction with water, and the stronger the brew becomes. Similarly, the smaller the fragments of minerals and rocks, the greater their surface area. The ratio of surface area to volume increases greatly as the average particle size decreases, as shown in Figure 16.3.
439
Carbon Dioxide, Weathering, and the Climate System
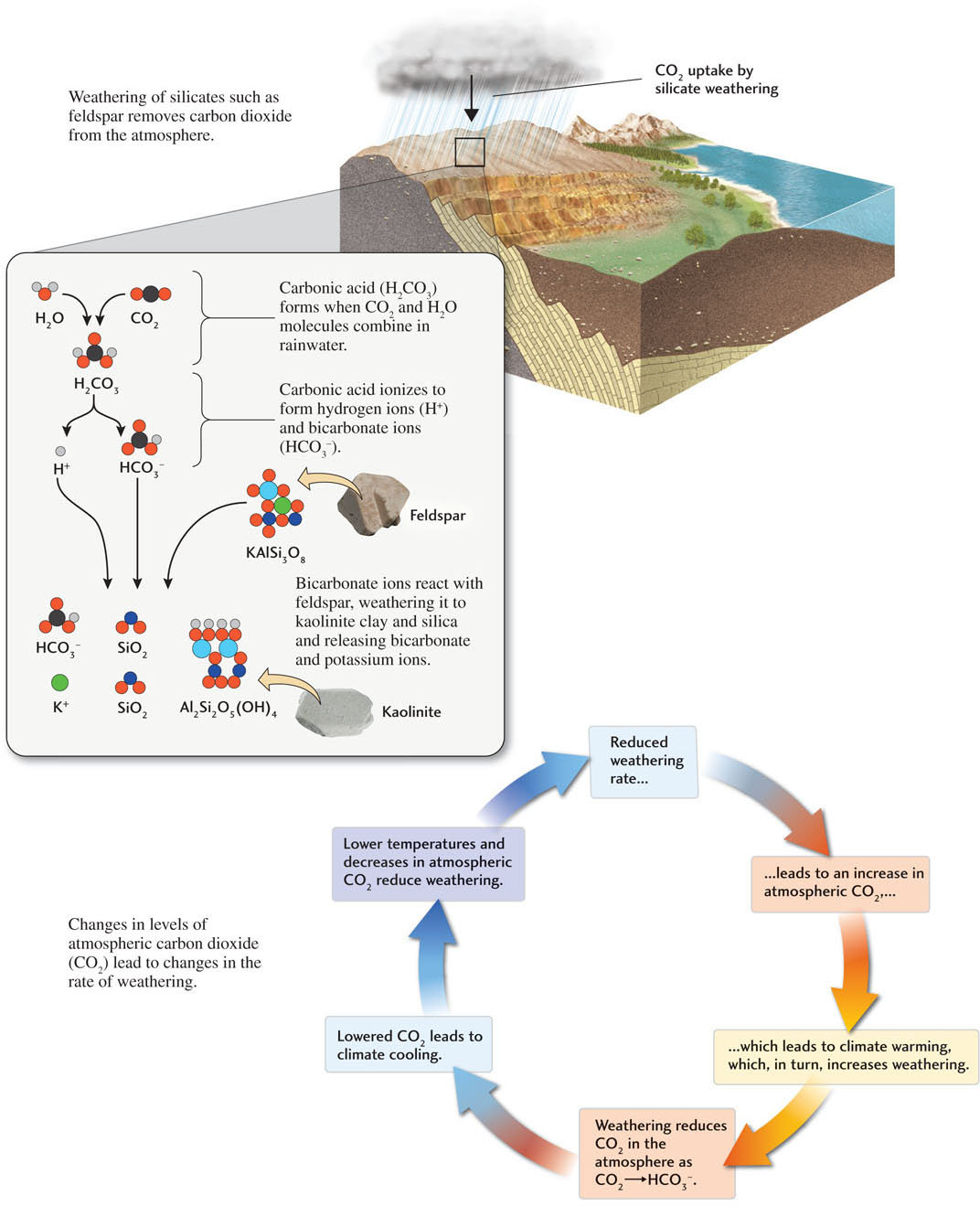
Carbon dioxide, like water, is involved in the chemical reactions of weathering. Thus, variation in the atmospheric concentration of CO2 leads to corresponding variation in the rate of weathering (Figure 16.4). Higher concentrations of CO2 in the atmosphere lead to higher concentrations in the soil, which accelerate the weathering of rocks. As we saw in Chapter 15, increases in atmospheric CO2, a greenhouse gas, make Earth’s climate warmer and thus promote weathering in that way as well. The weathering of calcium-rich rock, in turn, removes CO2 from the atmosphere, making global climates cooler. In this way, chemical weathering links the plate tectonic system to the climate system. As more and more CO2 is used up through weathering, the climate cools, and weathering decreases. As weathering decreases, the concentration of CO2 in the atmosphere builds up again, and the climate warms, thus continuing the cycle.
The Role of Carbon Dioxide in Weathering
The reaction of feldspar with pure water in a laboratory is an extremely slow process that would take thousands of years to break down even a small amount of feldspar completely. We can speed weathering by adding a strong acid (such as hydrochloric acid) to the water, in which case the feldspar will break down in a few days. An acid is a substance that releases hydrogen ions (H1) to a solution. A strong acid produces abundant hydrogen ions; a weak one, relatively few. The strong tendency of hydrogen ions to combine chemically with other substances makes acids excellent solvents.
On Earth’s surface, the most common acid—and the one most responsible for increasing weathering rates—is carbonic acid (H2CO3). This weak acid forms when carbon dioxide (CO2) gas from the atmosphere dissolves in rainwater:
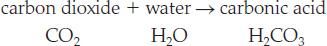
The amount of carbon dioxide dissolved in rainwater is small because the amount of CO2 gas in the atmosphere is small. About 0.03 percent of the molecules in Earth’s atmosphere are carbon dioxide. Thus, the amount of carbonic acid formed in rainwater is also very small, only about 0.0006 g/L.
As human activities increase the concentration of carbon dioxide in the atmosphere, the amount of carbonic acid in rainwater is increasing slightly. Acid rain accelerates weathering, but most of the acidity of acid rain comes not from carbon dioxide, but from sulfur dioxide and nitrogen gases, which react with water to form strong sulfuric and nitric acids, respectively. These acids promote weathering to a greater degree than carbonic acid does. Volcanoes and coastal wetlands emit gaseous forms of carbon, sulfur, and nitrogen into the atmosphere, but by far the largest source of these gases is industrial pollution.
Although rainwater contains only a relatively small amount of carbonic acid, that amount is enough to dissolve great quantities of rock over long periods. The chemical reaction for the weathering of feldspar is
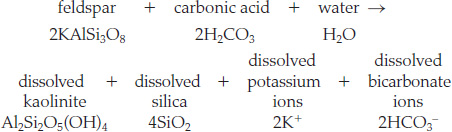
This simple weathering reaction illustrates the three main effects of chemical weathering on silicates:
- 1. It leaches, or dissolves away, cations and silica.
- 2. It hydrates, or adds water to, the minerals.
- 3. It makes solutions less acidic.
Specifically, the carbonic acid in rainwater helps to weather feldspar in the following way (see Figure 16.4):
 A small proportion of the carbonic acid molecules in rainwater ionize, forming hydrogen ions (H+) and bicarbonate ions (HCO3−), thus making the water slightly acidic.
A small proportion of the carbonic acid molecules in rainwater ionize, forming hydrogen ions (H+) and bicarbonate ions (HCO3−), thus making the water slightly acidic. The slightly acidic water dissolves potassium ions and silica from feldspar, leaving a residue of kaolinite, a solid clay. The hydrogen ions from the acidic water combine with the oxygen atoms of the feldspar to form the water in the kaolinite structure. The kaolinite becomes part of the soil or is carried away as the dissolved silica, potassium ions, and bicarbonate ions are carried away by rain and stream waters and are ultimately transported to the ocean.
The slightly acidic water dissolves potassium ions and silica from feldspar, leaving a residue of kaolinite, a solid clay. The hydrogen ions from the acidic water combine with the oxygen atoms of the feldspar to form the water in the kaolinite structure. The kaolinite becomes part of the soil or is carried away as the dissolved silica, potassium ions, and bicarbonate ions are carried away by rain and stream waters and are ultimately transported to the ocean.
440
441
The Role of Soil in Weathering
Now that we understand how acidic water weathers feldspar, we can better understand why feldspars on bare rock outcrops are better preserved than those buried in damp soils. The chemical reactions of weathering give us two separate but related clues to the factors responsible: the amount of water and the amount of acid available for those reactions. The exposed feldspar weathers only while the rock is moist with rainwater. During dry periods, the only moisture that touches bare rock is dew. The feldspar in damp soil, however, is constantly in contact with the small amounts of water retained in the pores between soil particles. Thus, feldspar weathers continuously in moist soil.
There is more acid in soil moisture than there is in falling rain. Rainwater carries its original carbonic acid into the soil. As water filters through the soil, it picks up additional carbonic acid and other acids produced by the roots of plants, by the many insects and other animals that live in the soil, and by the bacteria that degrade plant and animal remains. Recently, it was discovered that some bacteria release organic acids, even in waters hundreds of meters deep in the ground. These organic acids weather feldspar and other minerals in rocks below the surface. Bacterial respiration in soil may increase the soil’s carbon dioxide concentration to as much as 100 times the atmospheric concentration!
Rock weathers more rapidly in the tropics than in temperate and cold climates, mainly because plants and bacteria grow more quickly in warm, humid climates, contributing more of the carbonic acid and other acids that promote weathering. Additionally, most chemical reactions, weathering included, speed up with an increase in temperature.
The Role of Oxygen: From Iron Silicates to Iron Oxides
Iron is one of the eight most abundant chemical elements in Earth’s crust, but metallic iron, the element in its pure form, is rarely found in nature. It is present only in certain kinds of meteorites that fall to Earth from other places in the solar system. Most of the iron ores used for the production of iron and steel are formed by weathering. These ores are composed of iron oxide minerals originally produced by the weathering of iron-rich silicate minerals, such as pyroxene and olivine. The iron released by dissolution of the silicate minerals combines with oxygen from the atmosphere and hydrosphere to form iron oxide minerals.
The iron in minerals may be present in one of three forms: metallic iron, ferrous iron, or ferric iron. In the metallic iron found in meteorites (and in manufactured items), the iron atoms are uncharged: they have neither gained nor lost electrons by reacting with another element. In the ferrous iron (Fe2+) found in silicate minerals, the iron atoms have lost two of the electrons they have in the metallic form and have thus become ions. In the ferric iron (Fe3+) found in iron oxide minerals, the iron atoms have lost three electrons. The electrons lost by the iron are gained by oxygen atoms in a process called oxidation. Oxygen atoms in air and water oxidize ferrous iron to form ferric iron. Thus, all the iron oxides formed at Earth’s surface, the most abundant of which is hematite (Fe2O3), are ferric. Oxidation, like hydrolysis, is one of the important reactions of chemical weathering.
When an iron-rich silicate mineral such as pyroxene is exposed to water, its silicate structure breaks down, releasing silica and ferrous iron into the water. In solution, the ferrous iron is oxidized to the ferric form (Figure 16.5). The strong chemical bonds that form between ferric iron and oxygen make ferric iron insoluble in most natural surface waters. It therefore precipitates from solution, forming a solid ferric iron oxide. We are familiar with ferric iron oxide in another form: rust, which is produced when metallic iron in manufactured items is exposed to the atmosphere.
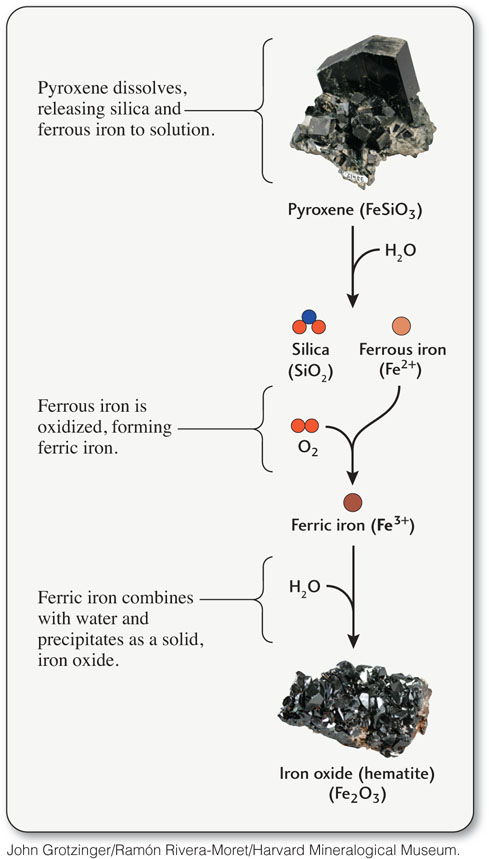
442
We can show this overall weathering reaction with the following example:
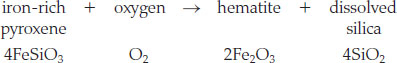
Although the equation does not show it explicitly, water is required for this reaction to proceed.
Iron-containing minerals, which are widespread, weather to the characteristic red and brown colors of oxidized iron (Figure 16.6). Iron oxides are found as coatings and encrustations that color soils and weathered surfaces of iron-containing rocks. The red soils of Georgia and other warm, humid regions are colored by iron oxides. In contrast, iron-rich minerals weather so slowly in polar regions that iron meteorites frozen in the ice of Antarctica are almost entirely unweathered.

Chemical Stability
Why do chemical weathering rates vary so widely among different minerals? Minerals weather at different rates because there are differences in their chemical stability in the presence of water at given temperatures.
Chemical stability is a measure of a substance’s tendency to retain its chemical identity rather than reacting spontaneously to become a different chemical substance. Chemical substances are stable or unstable in relation to a specific environment or set of conditions. Feldspar, for example, is stable under the conditions found deep in Earth’s crust (high temperatures and small amounts of water), but unstable under the conditions at Earth’s surface (lower temperatures and abundant water). Two characteristics of a mineral—its solubility and its rate of dissolution—help determine its chemical stability.
Solubility
The solubility of a mineral is measured by the amount of that mineral dissolved in water when the solution is saturated. Saturation is the point at which the water cannot hold any more of the dissolved substance. The higher a mineral’s solubility, the lower its chemical stability under most weathering conditions. Halite-containing evaporites, for example, are unstable under most weathering conditions. Their solubility is high (about 350 g/L), and they are leached from soil by even small amounts of water. Quartz, in contrast, is stable under most weathering conditions. Its solubility in water is very low (only about 0.008 g/L), and it is not easily leached from soil.
Rate of Dissolution
A mineral’s rate of dissolution is measured by the amount of that mineral that dissolves in an unsaturated solution in a given time. The faster the mineral dissolves, the less stable it is. Feldspar dissolves at a much faster rate than quartz, and primarily for that reason, it is less stable than quartz under weathering conditions.
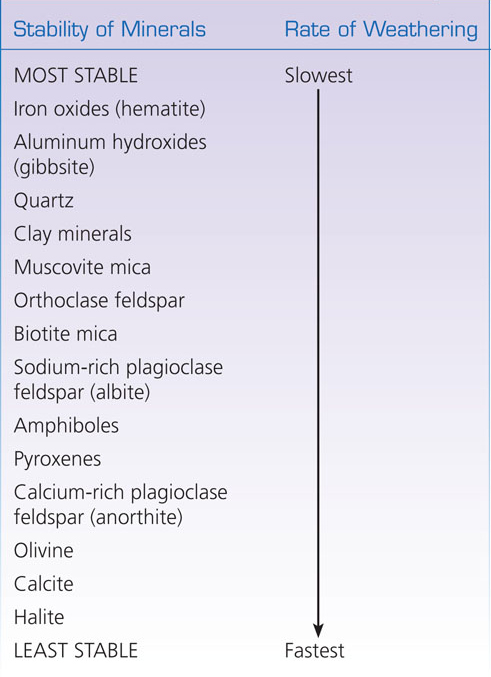
Relative Stabilities of Common Rock-Forming Minerals
The relative chemical stabilities of various minerals can be used to determine the intensity of weathering in a given area. In a tropical rain forest, only the most stable minerals will be left on an outcrop or in the soil, so we know that the weathering in that environment is intense. In an arid region such as the desert of North Africa, where weathering is minimal, alabaster (gypsum) monuments remain intact, as do many other unstable minerals. Table 16.2 shows the relative stabilities of all the common rock-forming minerals. Salt and carbonate minerals are the least stable, iron oxides the most stable.
443