The Formation of Minerals
The orderly forms of minerals result from the chemical bonds we have just described. Minerals can be viewed in two complementary ways: as assemblages of submicroscopic atoms organized in an ordered three-dimensional array, and as crystals that we can see with the naked eye. In this section, we examine the crystal structures of minerals and the conditions under which minerals form. Later in this chapter, we will see how the crystal structures of minerals are manifested in their physical properties.
The Atomic Structure of Minerals
Minerals form by the process of crystallization, in which the atoms of a gas or liquid come together in the proper chemical proportions and in the proper arrangement to form a solid substance. (Remember that the atoms in a mineral are arranged in an orderly three-dimensional array.) The bonding of carbon atoms in diamond, a covalently bonded mineral, is one example of crystallization. Under the very high pressures and temperatures in Earth’s mantle, carbon atoms bond together in tetrahedra, and each tetrahedron attaches to another, building up a regular three-dimensional structure from a great many atoms (see Figure 3.8). As a diamond crystal grows, it extends its tetrahedral structure in all directions, always adding new atoms in the proper geometric arrangement. Diamonds can be artificially synthesized from carbon under very high pressures and temperatures that mimic the conditions in Earth’s mantle.
The sodium and chloride ions that make up sodium chloride, an ionically bonded mineral, also crystallize in an orderly three-dimensional array. In Figure 3.4b, we can see the geometry of their arrangement, with each ion of one kind surrounded by six ions of the other kind in a series of cubic structures extending in three directions. We can think of ions as solid spheres, packed together in close-fitting structural units. Figure 3.4b also shows the relative sizes of the ions in NaCl. The relative sizes of the sodium and chloride ions allow them to fit together in a closely packed arrangement.
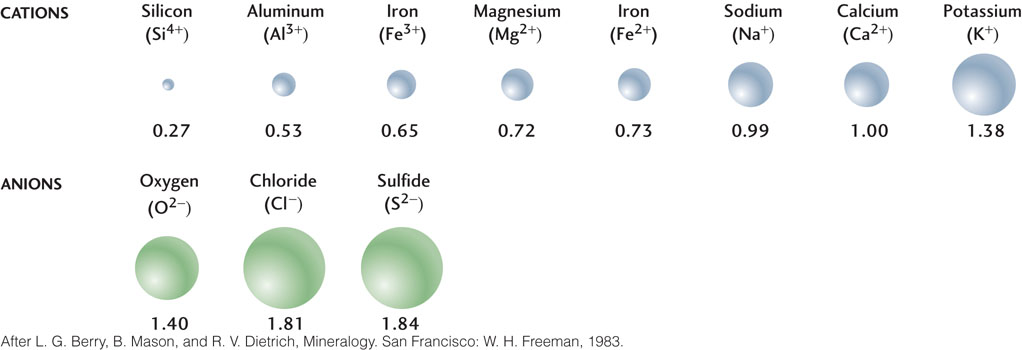
Many of the cations of abundant minerals are relatively small, while most anions—including the most common anion on Earth, oxygen (O2−)—are large (Figure 3.5). Because anions tend to be larger than cations, most of the space of a crystal is occupied by the anions, and the cations fit into the spaces between them. As a result, crystal structures are determined largely by how the anions are arranged and how the cations fit between them.
62
Cations of similar sizes and charges tend to substitute for one another and to form compounds having the same crystal structure but differing in chemical composition. Cation substitution is common in minerals that contain the silicate ion (SiO44−), such as olivine, which is abundant in many volcanic rocks. Iron (Fe2+) and magnesium (Mg2+) ions are similar to each other in size, and both have two positive charges, so they easily substitute for each other in the structure of olivine. The composition of pure magnesium olivine is Mg2SiO4; that of pure iron olivine is Fe2SiO4. The composition of olivine containing both iron and magnesium is given by the formula (Mg,Fe)2SiO4, which simply means that the number of iron and magnesium cations may vary, but their combined total (expressed as a subscript 2) in relation to the single SiO44− ion does not vary. The proportion of iron to magnesium is determined by the relative abundances of the two elements in the molten material from which the olivine crystallizes. Similarly, aluminum (Al3+) substitutes for silicon (Si4+) in many silicate minerals. Aluminum and silicon ions are similar enough in size that aluminum can take the place of silicon in many crystal structures. In this case, the difference in charge between aluminum (3+) and silicon (4+) ions is balanced by an increase in the number of other cations, such as sodium (1+).
The Crystallization of Minerals
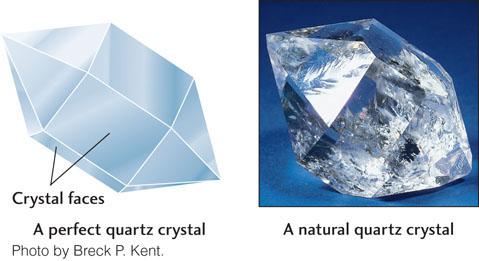
Crystallization starts with the formation of microscopic single crystals, orderly three-dimensional arrays of atoms in which the basic arrangement is repeated in all directions. The boundaries of crystals are natural flat (planar) surfaces called crystal faces (Figure 3.6). The crystal faces of a mineral are the external expression of the mineral’s internal atomic structure. Figure 3.7 pairs a drawing of a perfect quartz crystal with a photograph of the actual mineral. The six-sided (hexagonal) shape of the quartz crystal corresponds to its hexagonal internal atomic structure.

During crystallization, the initially microscopic crystals grow larger, maintaining their crystal faces as long as they are free to grow. Large crystals with well-defined faces form when growth is slow and steady and space is adequate to allow growth without interference from other crystals nearby (Figure 3.8). For this reason, most large mineral crystals form in open spaces in rocks, such as fractures or cavities.

63
More often, however, the spaces between growing crystals fill in, or crystallization proceeds rapidly. Crystals then grow over one another and coalesce to become a solid mass of crystalline particles, or grains. In this case, few or no grains show crystal faces. Large crystals that can be seen with the naked eye are relatively unusual, but many minerals in rocks display crystal faces that can be seen under a microscope.
Unlike minerals, glassy materials—which solidify from liquids so quickly that they lack any internal atomic order—do not form crystals with planar faces. Instead, they are found as masses with curved, irregular surfaces. The most common natural glass is volcanic glass.
How Do Minerals Form?
Lowering the temperature of a liquid below its freezing point is one way to start the process of crystallization. In water, for example, 0°C is the temperature below which crystals of ice—a mineral—start to form. Similarly, a magma—a mass of hot, molten liquid rock—crystallizes into solid minerals when it cools. As a magma falls below its melting point, which may be higher than 1000°C depending on the elements it contains, crystals of silicate minerals such as olivine or feldspar begin to form. (Geologists usually refer to melting points of magmas rather than freezing points, because freezing implies cold.)
Crystallization can also occur as liquids evaporate from a solution. A solution is a homogeneous mixture of one chemical substance with another, such as salt and water. As the water evaporates from a salt solution, the concentration of salt eventually gets so high that the solution can hold no more salt and is said to be saturated. If evaporation continues, the salt starts to precipitate, or drop out of solution as crystals. Deposits of table salt, or halite, form under just these conditions when seawater evaporates to the point of saturation in some hot, arid bays or arms of the ocean (Figure 3.9).

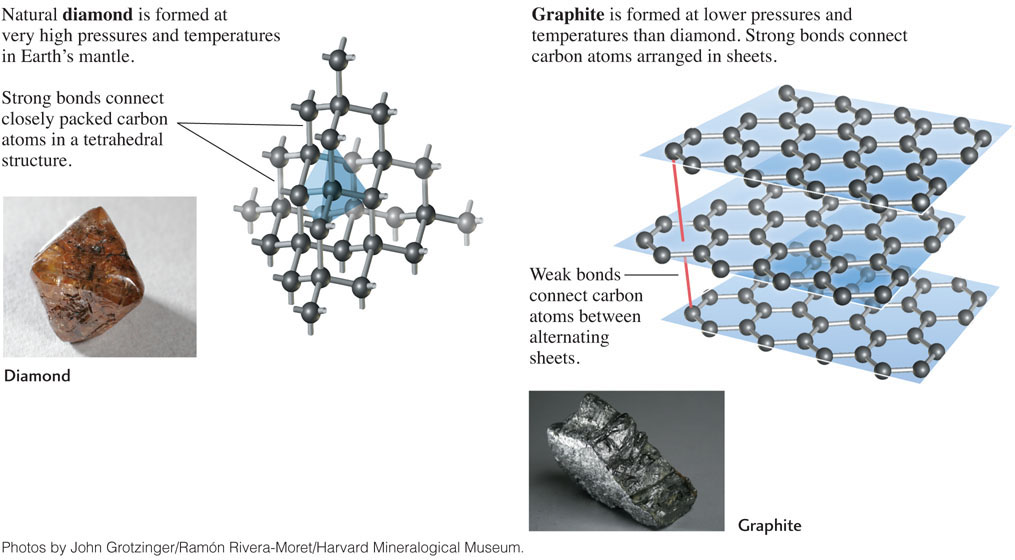
Diamond and graphite (the material used as the “lead” in pencils) exemplify the dramatic effects that temperature and pressure can have on mineral formation. These two minerals are polymorphs, minerals with alternative structures formed from the same chemical element or compound (Figure 3.10). They are both formed from carbon, but have different crystal structures and very different appearances. From experimentation and geologic observation, we know that diamond forms and remains stable at the very high pressures and temperatures found in Earth’s mantle. High pressures force the atoms in diamond into a closely packed structure. Diamond therefore has a higher density (mass per unit volume, usually expressed in grams per cubic centimeter, g/cm3) than graphite, which is less closely packed: diamond has a density of 3.5 g/cm3, while that of graphite is only 2.1 g/cm3. Graphite forms and is stable at moderate pressures and temperatures, such as those in Earth’s crust.
64
Low temperatures can also produce close packing of atoms. Quartz and cristobalite, for example, are polymorphs of silica (SiO2). Quartz forms at low temperatures and is relatively dense (2.7 g/cm3). Cristobalite, which forms at higher temperatures, has a more open structure and is therefore less dense (2.3 g/cm3).