Surface Processes of the Rock Cycle
Sediments, and the sedimentary rocks formed from them, are produced by the surface processes of the rock cycle. These processes act on rocks after they have been moved from Earth’s interior to its surface and uplifted by mountain building and before they are returned to Earth’s interior by subduction. They move materials from a source area, where sediment particles are created, to a sink area, where they are deposited in layers. The path that sediment particles follow from source to sink may be a very long one—one that involves several important processes resulting from interactions between the plate tectonic and climate systems.
Let’s look at the role of the Mississippi River in a typical sedimentary process. Plate movement lifts up rocks in the Rocky Mountains. Rainfall in those mountains—a source area—causes weathering of the rocks there. If precipitation increases in the mountains, weathering also increases. Faster weathering produces more sediment to be released into the river and transported downhill and downstream. At the same time, if the flow in the river also increases because of the higher rainfall, transportation of sediment down the length of the river increases, and the volume of sediment delivered to sink areas—sites of deposition, also known as sedimentary basins—in the Mississippi delta and the Gulf of Mexico increases as well. In these sedimentary basins, the sediments pile up on top of one another—layer after layer—and are eventually buried deep in Earth’s crust, to depths where they may become filled with valuable oil and natural gas.
The surface processes of the rock cycle that are important in the formation of sedimentary rocks are reviewed in Figure 5.1 and summarized here:
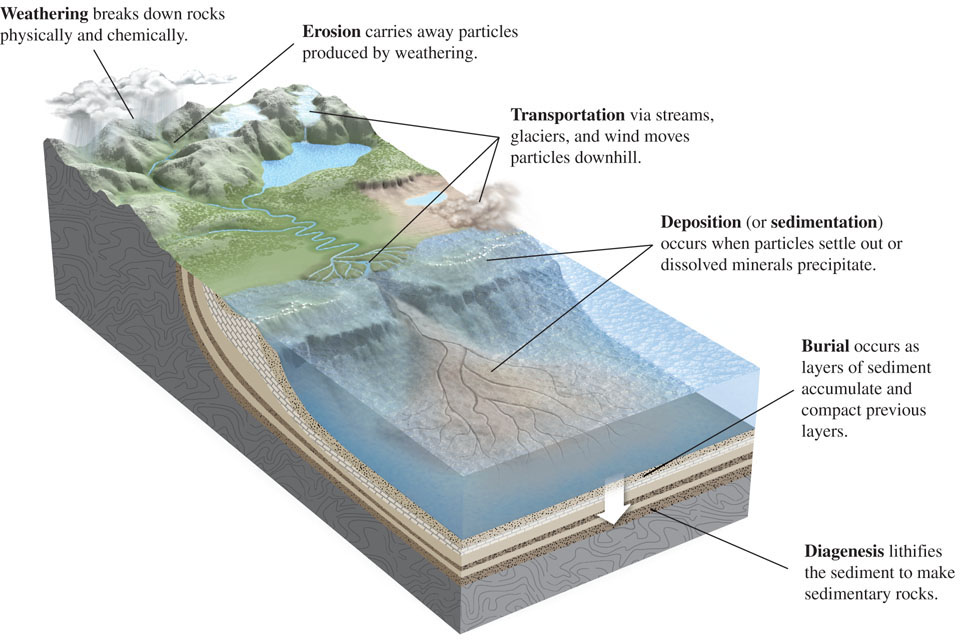
 Weathering is the general process by which rocks are broken down at Earth’s surface to produce sediment particles. There are two types of weathering. Physical weathering takes place when solid rock is fragmented by mechanical processes, such as freezing and thawing or wedging by tree roots (Figure 5.2), that do not change its chemical composition. The rubble of broken stone often seen at the tops of mountains and hills is primarily the result of physical weathering. Chemical weathering refers to processes by which the minerals in a rock are chemically altered or dissolved. The blurring or disappearance of lettering on old gravestones and monuments is caused mainly by chemical weathering.
Weathering is the general process by which rocks are broken down at Earth’s surface to produce sediment particles. There are two types of weathering. Physical weathering takes place when solid rock is fragmented by mechanical processes, such as freezing and thawing or wedging by tree roots (Figure 5.2), that do not change its chemical composition. The rubble of broken stone often seen at the tops of mountains and hills is primarily the result of physical weathering. Chemical weathering refers to processes by which the minerals in a rock are chemically altered or dissolved. The blurring or disappearance of lettering on old gravestones and monuments is caused mainly by chemical weathering. Figure 5.2 Plant roots contribute to physical weathering by penetrating fractures and wedging rocks apart.
Figure 5.2 Plant roots contribute to physical weathering by penetrating fractures and wedging rocks apart. Erosion refers to processes that dislodge particles of rock produced by weathering and move them away from the source area. Erosion occurs most commonly when rainwater runs downhill.
Erosion refers to processes that dislodge particles of rock produced by weathering and move them away from the source area. Erosion occurs most commonly when rainwater runs downhill. Transportation refers to processes by which sediment particles are moved to sink areas. Transportation occurs when water, wind, or the moving ice of glaciers transport particles to new locations downhill or downstream.
Transportation refers to processes by which sediment particles are moved to sink areas. Transportation occurs when water, wind, or the moving ice of glaciers transport particles to new locations downhill or downstream. Deposition (also called sedimentation) refers to processes by which sediment particles settle out as water currents slow, winds die down, or glacier edges melt to form layers of sediment in sink areas. In aquatic environments, particles settle out, chemical precipitates form and are deposited, and the bodies and shells of dead organisms are broken up and deposited.
Deposition (also called sedimentation) refers to processes by which sediment particles settle out as water currents slow, winds die down, or glacier edges melt to form layers of sediment in sink areas. In aquatic environments, particles settle out, chemical precipitates form and are deposited, and the bodies and shells of dead organisms are broken up and deposited. Burial occurs as layers of sediment accumulate in sink areas on top of older, previously deposited sediments, which are compacted and progressively buried deep within a sedimentary basin. These sediments will remain at depth, as part of Earth’s crust, until they are either uplifted again or subducted by plate tectonic processes.
Burial occurs as layers of sediment accumulate in sink areas on top of older, previously deposited sediments, which are compacted and progressively buried deep within a sedimentary basin. These sediments will remain at depth, as part of Earth’s crust, until they are either uplifted again or subducted by plate tectonic processes. Diagenesis refers to the physical and chemical changes—caused by pressure, heat, and chemical reactions—by which sediments buried within sedimentary basins are lithified, or converted into sedimentary rock.
Diagenesis refers to the physical and chemical changes—caused by pressure, heat, and chemical reactions—by which sediments buried within sedimentary basins are lithified, or converted into sedimentary rock.
Weathering and Erosion: The Source of Sediments
Chemical and physical weathering reinforce each other. Chemical weathering weakens rocks and makes them more susceptible to fragmentation. The smaller the fragments produced by physical weathering, the greater the surface area exposed to chemical weathering. Together, physical and chemical weathering of rock create both solid particles and dissolved products, and erosion carries them away. These end products can be classified as either siliciclastic sediments or chemical and biological sediments.
Siliciclastic Sediments
Physical and chemical weathering of preexisting rocks forms clastic particles that are transported and deposited as sediments. Clastic particles range in size from boulders to particles of sand, silt, and clay. They also vary widely in shape. Natural breakage along bedding planes and fractures in the parent rock determine the shapes of boulders, cobbles, and pebbles. Sand grains are the remnants of individual crystals formerly interlocked in parent rock, and their shapes tend to reflect the shapes of those crystals.
118
Most clastic particles are produced by the weathering of common rocks composed largely of silicate minerals, so sediments formed from these particles are called siliciclastic sediments. The mixture of minerals in siliciclastic sediments varies. Minerals such as quartz resist weathering and thus are found chemically unaltered in siliciclastic sediments. These sediments may also contain partly altered fragments of minerals, such as feldspar, that are less resistant to weathering and therefore less stable. Still other minerals in siliciclastic sediments, such as clay minerals, are newly formed by chemical weathering. Varying intensities of weathering can produce different sets of minerals in sediments derived from the same parent rock. Where weathering is intense, the sediment will contain only clastic particles made of chemically stable minerals, mixed with clay minerals. Where weathering is slight, many minerals that are unstable under land surface conditions will survive as clastic particles in the sediment. Table 5.1 shows three possible sets of minerals in sediments derived from a typical granite outcrop.
| Intensity of Weathering | ||
|---|---|---|
| Low | Medium | High |
| Quartz | Quartz | Quartz |
| Feldspar | Feldspar | Clay minerals |
| Mica | Mica | |
| Pyroxene | Clay minerals | |
| Amphibole | ||
Chemical and Biological Sediments
Chemical weathering produces dissolved ions and molecules that accumulate in the waters of soils, rivers, lakes, and oceans. Chemical and biological reactions then precipitate these substances to form chemical and biological sediments. We distinguish between chemical and biological sediments mainly for convenience; in practice, many chemical and biological sediments overlap. Chemical sediments form at or near their place of deposition. The evaporation of seawater, for example, often leads to the precipitation of gypsum or halite (Figure 5.3).

Biological sediments also form near their place of deposition, but they are the result of mineral precipitation by organisms. Some organisms, such as mollusks and corals, precipitate minerals as they grow. After the organisms die, their shells or skeletons accumulate on the seafloor as sediments. In these cases, the organism directly controls mineral precipitation. However, in a second but equally important process, organisms control mineral precipitation only indirectly. Instead of taking up minerals from the water to form a shell, these organisms change the surrounding environment so that mineral precipitation occurs on the outside of the organism, or even away from the organism. Certain microorganisms are thought to enable the precipitation of pyrite (an iron sulfide mineral) in this way (see Chapter 11).
119
In shallow marine environments, directly precipitated biological sediments consist of layers of particles, such as whole or fragmented shells of marine organisms (Figure 5.4). Many different types of organisms, ranging from corals to clams to algae, may contribute their shells. Sometimes the shells are transported, further broken up, and deposited as bioclastic sediments. These shallow-water sediments consist predominantly of two calcium carbonate minerals, calcite and aragonite, in variable proportions. Other minerals, such as phosphates and sulfates, are only locally abundant in bioclastic sediments.

In the deep sea, biological sediments are made up of the shells of only a few kinds of planktonic organisms. Most of these organisms secrete shells composed primarily of calcite and aragonite, but a few species form silica shells, which are precipitated broadly over some parts of the deep seafloor. Because these biological particles accumulate in very deep water, where agitation by sediment-transporting currents is uncommon, they rarely form bioclastic sediments.
Transportation and Deposition: The Downhill Journey to Sedimentary Basins
After clastic particles and dissolved ions have been formed by weathering and dislodged by erosion, they start their journey to a sedimentary basin. This journey may be very long; for example, as we have seen, it might span the thousands of kilometers from the tributaries of the Mississippi River in the Rocky Mountains to the wetlands of the Mississippi delta.
Most agents of sediment transport carry sediments on a one-way trip downhill. Rocks falling from a cliff, a river flowing to the ocean carrying a load of sand, and glacial ice slowly dragging boulders downhill are all responses to gravity. Although wind may blow material from a low elevation to a higher one, in the long run the effects of gravity prevail. When a windblown particle drops into the ocean and settles through the water, it is trapped. It can be picked up again only by an ocean current, which can transport it to and deposit it in another site on the seafloor. Ocean currents transport sediments over shorter distances than do big rivers on land, and the short transportation distances of chemical and biological sediments contrast with the much greater distances over which siliciclastic sediments are transported. But eventually, all sediment transportation paths, as simple or complicated as they may be, lead downhill into a sedimentary basin.
Currents as Transport Agents
Most sediments are transported by currents of air or water. The enormous quantities of all kinds of sediments found in the oceans result primarily from the transport capacities of rivers, which annually carry a solid and dissolved sediment load of about 25 billion tons (25 × 1015 g) (Figure 5.5). Air currents—winds—move sediments, too, but in far smaller quantities than rivers or ocean currents. As particles are lifted into the air or water, the current carries them downwind or downstream. The stronger the current—that is, the faster it flows—the larger the particles it can transport.
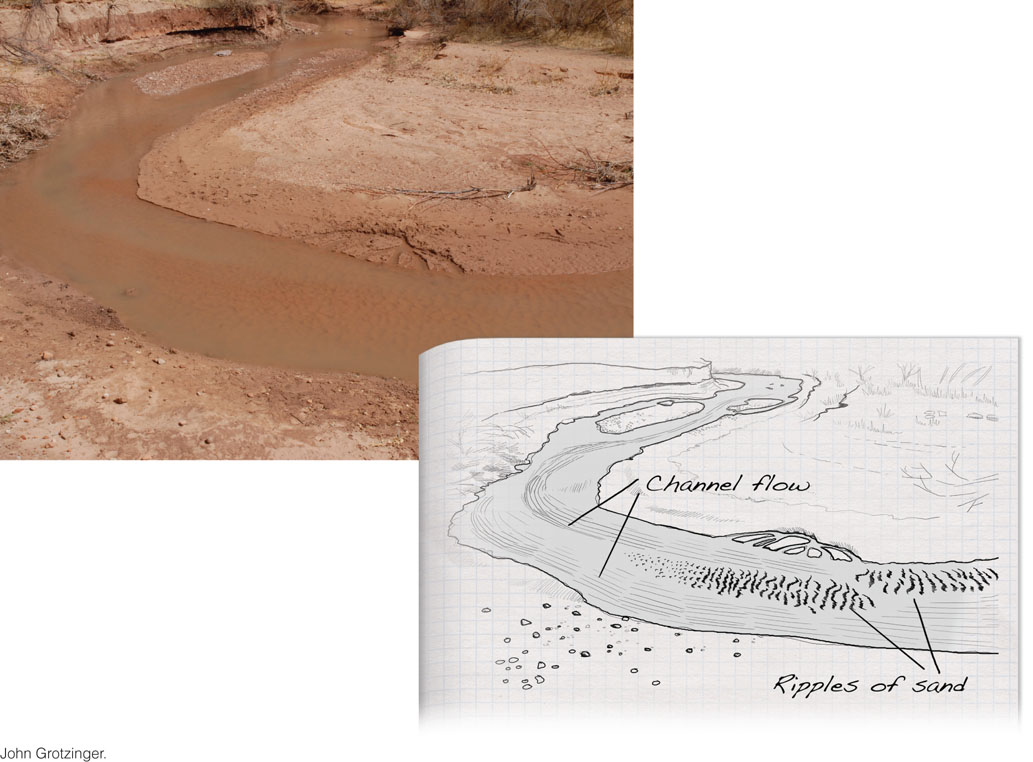
Current Strength, Particle Size, and Sorting
Deposition starts where transportation stops. For clastic particles, gravity is the driving force of deposition. The tendency of particles to settle under the pull of gravity works against a current’s ability to carry them. A particle’s settling velocity is proportional to its density and its size (see Chapter 4, Practicing Geology). Because all clastic particles have roughly the same density, we use particle size as the best indicator of how quickly a particle will settle. (We will take a more specific look at particle size categories later in this chapter.) In water, large particles settle faster than small ones. This is also true in air, but the difference is much smaller.
120
Current strength, which is directly related to current velocity, determines the size of the particles deposited in a particular place. As a wind or water current begins to slow, it can no longer keep the largest particles suspended, and those particles settle. As the current slows even more, smaller particles settle. When the current stops completely, even the smallest particles settle. Currents segregate particles in the following ways:
 Strong currents (faster than 50 cm/s) carry gravel (which includes boulders, cobbles, and pebbles), along with an abundant supply of smaller particles. Such currents are common in swiftly flowing streams in mountainous terrain, where erosion is rapid. Beach gravels are deposited where ocean waves erode rocky shores.
Strong currents (faster than 50 cm/s) carry gravel (which includes boulders, cobbles, and pebbles), along with an abundant supply of smaller particles. Such currents are common in swiftly flowing streams in mountainous terrain, where erosion is rapid. Beach gravels are deposited where ocean waves erode rocky shores. Moderately strong currents (20–50 cm/s) lay down sand beds. Currents of moderate strength are common in most rivers, which carry and deposit sand in their channels. Rapidly flowing floodwaters may spread sand over the width of a river valley. Waves and currents deposit sand on beaches and in the ocean. Winds also blow and deposit sand, especially in deserts. However, because air is much less dense than water, much higher current velocities are required for it to move sediments of the same size and density.
Moderately strong currents (20–50 cm/s) lay down sand beds. Currents of moderate strength are common in most rivers, which carry and deposit sand in their channels. Rapidly flowing floodwaters may spread sand over the width of a river valley. Waves and currents deposit sand on beaches and in the ocean. Winds also blow and deposit sand, especially in deserts. However, because air is much less dense than water, much higher current velocities are required for it to move sediments of the same size and density. Weak currents (slower than 20 cm/s) carry muds composed of the finest clastic particles (silt and clay). Weak currents are found on the floor of a river valley when floodwaters recede slowly or stop flowing entirely. In the ocean, muds are generally deposited some distance from shore, where currents are too slow to keep even fine particles in suspension. Much of the floor of the open ocean is covered with mud particles originally transported by surface waves and currents or by wind. These particles slowly settle to depths where currents and waves are stilled and, ultimately, all the way to the bottom of the ocean.
Weak currents (slower than 20 cm/s) carry muds composed of the finest clastic particles (silt and clay). Weak currents are found on the floor of a river valley when floodwaters recede slowly or stop flowing entirely. In the ocean, muds are generally deposited some distance from shore, where currents are too slow to keep even fine particles in suspension. Much of the floor of the open ocean is covered with mud particles originally transported by surface waves and currents or by wind. These particles slowly settle to depths where currents and waves are stilled and, ultimately, all the way to the bottom of the ocean.
As you can see, currents may begin by carrying particles of widely varying sizes, which then become separated as the strength of the current changes. A strong, fast current may lay down a bed of gravel while keeping sand and mud in suspension. If the current weakens and slows, it will lay down a bed of sand on top of the gravel. If the current then stops altogether, it will deposit a layer of mud on top of the sand bed. This tendency for variations in current velocity to segregate sediments according to size is called sorting. A well-sorted sediment consists mostly of particles of a uniform size. A poorly sorted sediment contains particles of many sizes (Figure 5.6).
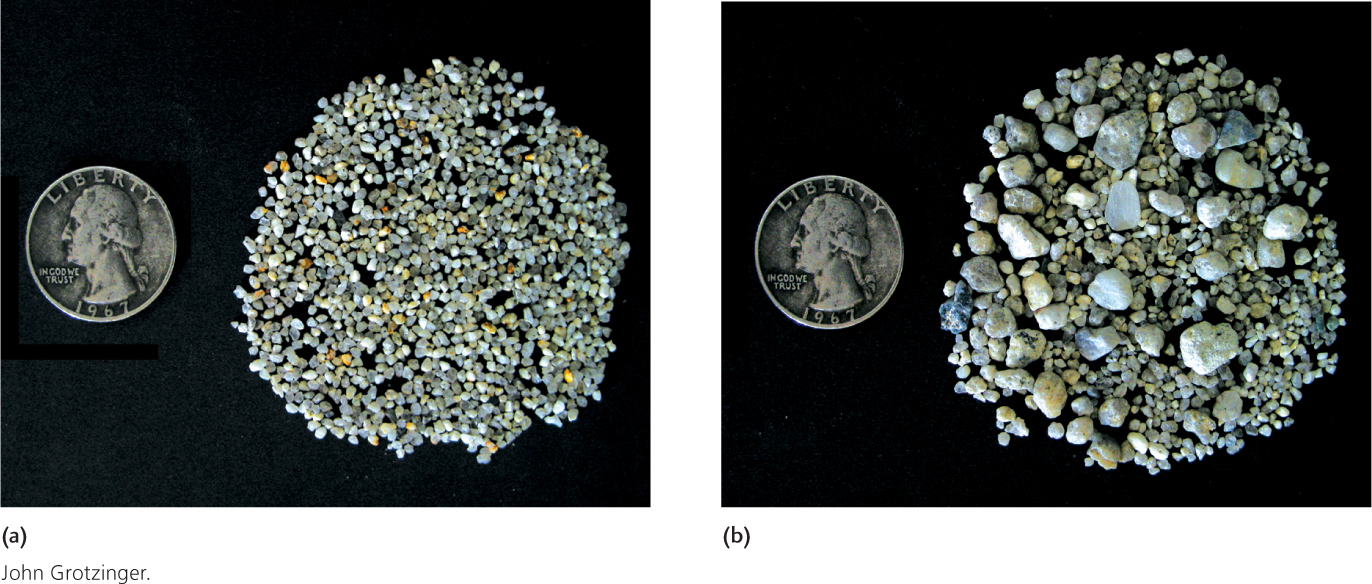
121
As cobbles, pebbles, and large sand grains are being transported by water or air currents, they tumble and strike one another or rub against the underlying rock. The resulting abrasion affects the particles in two ways: it reduces their size, and it rounds off their rough edges (Figure 5.7). These effects apply mostly to the larger particles; smaller sand grains and silt undergo little abrasion.
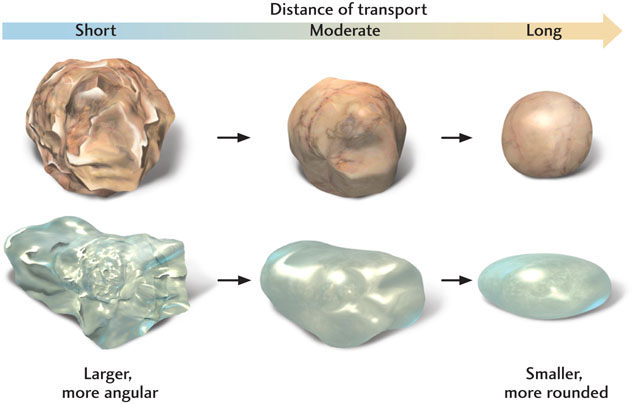
Particles are generally transported intermittently rather than steadily. A river may transport large quantities of sand and gravel when it floods, then drop them as the flood recedes, only to pick them up again and carry them even farther in the next flood. Similarly, strong winds may carry large amounts of dust for a few days, then die down and deposit the dust as a layer of sediment. The strong tidal currents along some shorelines may transport broken shell fragments to places farther offshore and drop them there.
The total time it takes for clastic particles to be transported may be many hundreds or thousands of years, depending on the distance to the final sedimentary basin and the number of stops along the way. Clastic particles eroded by the headwaters of the Missouri River in the mountains of western Montana, for example, take hundreds of years to travel the 3200 km down the Missouri and Mississippi rivers to the Gulf of Mexico.
122
Oceans as Chemical Mixing Vats
The driving force of chemical and biological sedimentation is precipitation rather than gravity. Substances dissolved in water by chemical weathering are carried along with the water. These materials are part of the aqueous solution itself, so gravity cannot cause them to settle out. As the dissolved materials are carried down rivers, they ultimately enter the ocean.
Oceans may be thought of as huge chemical mixing vats. Rivers, rain, wind, and glaciers constantly bring in dissolved materials. Smaller quantities of dissolved materials enter the oceans through hydrothermal chemical reactions between seawater and hot basalt at mid-ocean ridges. The oceans lose water continuously by evaporation at the surface. The inflow and outflow of water are so exactly balanced that the amount of water in the oceans remains constant over such geologically short times as years, decades, or even centuries. Over a time scale of thousands to millions of years, however, the balance may shift. During the most recent ice age, for example, significant quantities of seawater were converted into glacial ice, and sea level was drawn down by more than 100 m.
The entry and exit of dissolved materials, too, are balanced. Each of the many dissolved components of seawater participates in some chemical or biological reaction that eventually precipitates it out of the water and onto the seafloor. As a result, the ocean’s salinity—the total amount of dissolved material in a given volume of water—remains constant. Totaled over the world ocean, mineral precipitation balances the total inflow of dissolved material—yet another way in which the Earth system maintains balance.
We can better understand this chemical balance by considering the element calcium. Calcium is a component of the most abundant biological precipitate formed in the oceans: calcium carbonate (CaCO3). On land, calcium dissolves when limestone and calcium-containing silicate minerals, such as some feldspars and pyroxenes, are weathered, and that calcium is transported to the ocean as dissolved calcium ions (Ca2+). There, a wide variety of marine organisms take up calcium ions and combine them with carbonate ions (CO32−), also present in seawater, to form their calcium carbonate shells. Thus, the calcium that entered the ocean as dissolved ions leaves it as solid sediment particles when the organisms die and their shells settle to the seafloor and accumulate there as calcium carbonate sediments. Ultimately, the calcium carbonate sediments will be buried and transformed into limestone. The chemical balance that keeps the concentrations of calcium dissolved in the ocean constant is thus controlled in part by the activities of organisms.
Nonbiological mechanisms also maintain chemical balance in the oceans. For example, sodium ions (Na+) transported to the oceans react chemically with chloride ions (Cl−) to form the precipitate sodium chloride (NaCl). This happens when evaporation raises the concentrations of sodium and chloride ions past the point of saturation. As we saw in Chapter 3, minerals precipitate when solutions become so saturated with dissolved materials that they can hold no more. The intense evaporation required to crystallize sodium chloride may take place in warm, shallow arms of the sea or in saline lakes.