Causes of Metamorphism
Sediments and sedimentary rocks are products of Earth’s surface environments, whereas igneous rocks are products of the magmas that originate in the lower crust and mantle. Metamorphic rocks are the products of processes acting on rocks at depths ranging from the upper to the lower crust.
When a rock is subjected to significant changes in temperature or pressure, it will, given enough time—short by geologic standards, but usually a million years or more—undergo changes in its chemical composition, mineralogy, and texture, or all three, until it is in equilibrium with the new temperature and pressure. A limestone filled with fossils, for example, may be transformed into a white marble in which no trace of fossils remains. The mineral and chemical composition of the rock may be unaltered, but its texture may have changed drastically, from small calcite crystals to large, interlocked calcite crystals that erase such former features as fossils. Shale, a well-bedded sedimentary rock so fine-grained that no individual crystal can be seen with the naked eye, may become schist, in which the original bedding is obscured and the texture is dominated by large crystals of mica. In this case, both mineralogy and texture have changed, but the overall chemical composition of the rock has remained the same.
Most metamorphic rocks are formed at depths of 10 to 30 km, in the middle to lower half of the crust. Only later are those rocks exhumed, or transported back to Earth’s surface, where they may be exposed as outcrops. But metamorphism can also occur at Earth’s surface. We can see metamorphic changes, for example, in the baked surfaces of soils and sediments just beneath volcanic lava flows.
The heat and pressure in Earth’s interior and its fluid composition are the three principal factors that drive metamorphism. In much of Earth’s crust, the temperature increases at a rate of 30°C per kilometer of depth, although that rate varies considerably among different regions, as we will see shortly. Thus, at a depth of 15 km, the temperature will be about 450°C—much higher than the average temperature at Earth’s surface, which ranges from 10°C to 20°C in most regions. The contribution of pressure is the result of vertically oriented forces exerted by the weight of overlying rocks as well as horizontally oriented forces developed as the rocks are deformed by plate tectonic processes. The average pressure at a depth of 15 km amounts to about 4000 times the pressure at the surface.
As high as these temperatures and pressures may seem, they are only in the middle range of conditions for metamorphism, as Figure 6.1 shows. A rock’s metamorphic grade reflects the temperatures and pressures it was subjected to during metamorphism. We refer to metamorphic rocks formed under the lower temperatures and pressures of shallower crustal regions as low-grade metamorphic rocks and those formed under the higher temperatures and pressures at greater depths as high-grade metamorphic rocks.
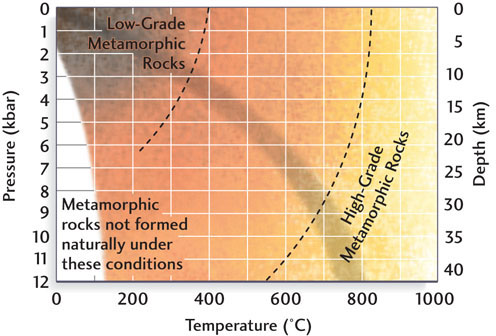
As the grade of metamorphism changes, the assemblages of minerals within metamorphic rocks also change. Some silicate minerals are found mostly in metamorphic rocks: these minerals include kyanite, andalusite, sillimanite, staurolite, garnet, and epidote. Geologists use distinctive textures as well as mineral composition to help guide their studies of metamorphic rocks.
151
The Role of Temperature
Heat can transform a rock’s chemical composition, mineralogy, and texture by breaking chemical bonds and altering the existing crystal structures of the rock. When rock is moved from Earth’s surface to its interior, where temperatures are higher, the rock adjusts to the new temperature. Its atoms and ions recrystallize, linking up in new arrangements and creating new mineral assemblages. Many new crystals grow larger than the crystals in the original rock.
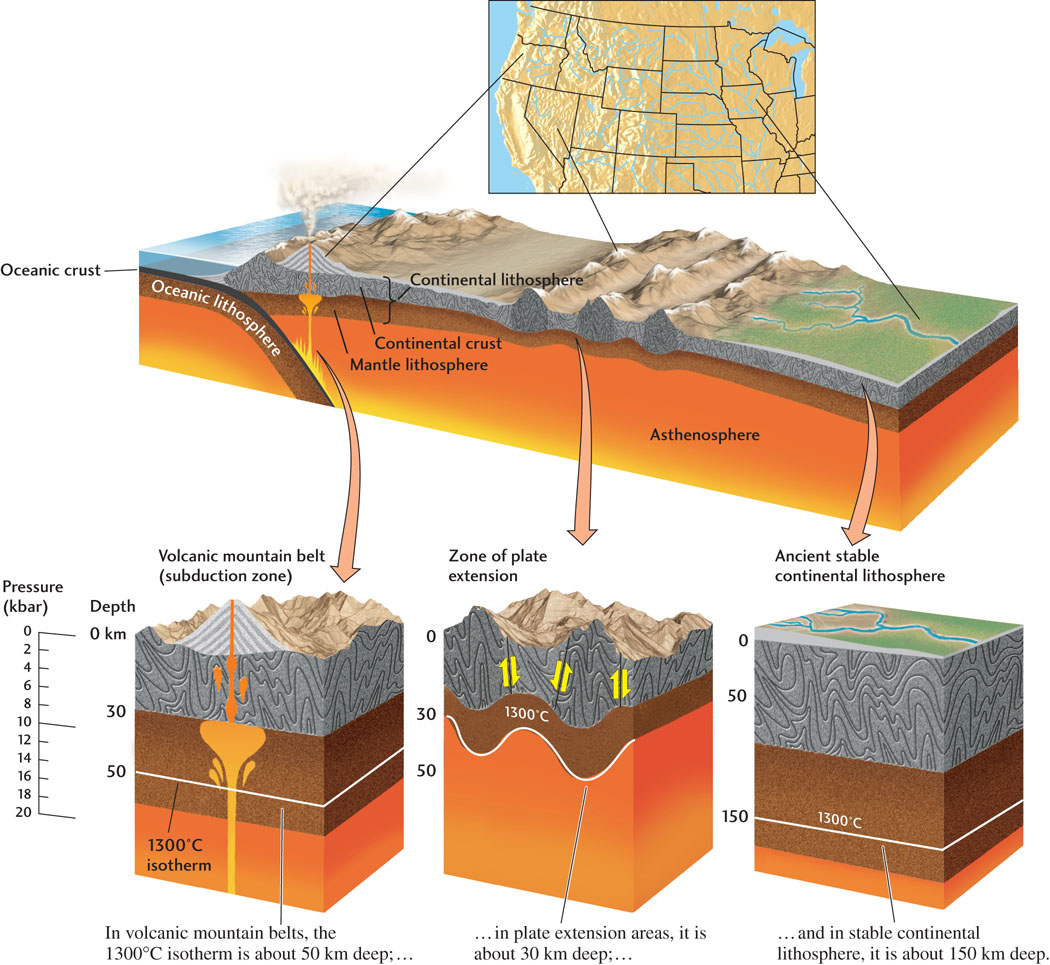
The increase in temperature with increasing depth in Earth’s interior is called the geothermal gradient. The geothermal gradient varies among plate tectonic settings, but on average it is about 30°C per kilometer of depth. In areas where the continental lithosphere has been stretched and thinned, such as Nevada’s Great Basin, the geothermal gradient is steep (for example, 50°C per kilometer of depth). In areas where the continental lithosphere is old and thick, such as central North America, the geothermal gradient is shallow (for example, 20°C per kilometer of depth) (Figure 6.2).
Because different minerals crystallize and remain stable at different temperatures, we can use a rock’s mineral composition as a kind of geothermometer to gauge the temperature at which it formed. For example, as sedimentary rocks containing clay minerals are buried deeper and deeper, the clay minerals begin to recrystallize and form new minerals, such as micas. With additional burial at greater depths and temperatures, the micas become unstable and begin to recrystallize into new minerals, such as garnet.
Plate tectonic processes such as subduction and continent-continent collision, which transport rocks and sediments into the hot depths of the crust, are the mechanisms that form most metamorphic rocks. In addition, limited metamorphism may occur where rocks are subjected to elevated temperatures near igneous intrusions. The heat is locally intense, but does not penetrate deeply; thus, the intrusions can metamorphose the surrounding country rock, but the effect is local in extent.
152
The Role of Pressure
Pressure, like temperature, changes a rock’s chemical composition, mineralogy, and texture. Solid rock is subjected to two basic kinds of pressure, also called stress:
- 1. Confining pressure is a general force applied equally in all directions, like the pressure a swimmer feels under water. Just as a swimmer feels greater confining pressure when diving to greater depths, a rock descending to greater depths in Earth’s interior is subjected to progressively increasing confining pressure in proportion to the weight of the overlying mass.
- 2. Directed pressure, or differential stress, is force exerted in a particular direction, as when you squeeze a ball of clay between your thumb and forefinger. Directed pressure is usually concentrated within particular zones or along discrete planes.
The compressive force exerted where lithospheric plates converge is a form of directed pressure, and it results in deformation of the rocks near the plate boundary. Heat reduces the strength of a rock, so directed pressure is likely to cause severe folding and other forms of ductile deformation, as well as metamorphism, where temperatures are high. Rocks subjected to differential stress may be severely distorted, becoming flattened in the direction the force is applied and elongated in the direction perpendicular to the force (Figure 6.3).

The minerals in a rock under pressure may be compressed, elongated, or rotated to line up in a particular direction, depending on the kind of stress applied to the rock. Thus, directed pressure guides the shape and orientation of the new crystals formed as minerals recrystallize under the influence of both heat and pressure. During the recrystallization of micas, for example, the crystals grow with the planes of their sheet silicate structures aligned perpendicular to the directed stress. The rock may develop a banded pattern as minerals of different compositions are segregated into separate planes.
Marble owes its remarkable strength to this recrystallization process. When limestone, a sedimentary rock, is heated to the very high temperatures that cause it to recrystallize, the original minerals and crystals become reoriented and tightly interlocked to form a very strong structure with no planes of weakness.
The pressure to which rock is subjected deep in Earth’s crust is related to both the thickness and the density of the overlying rocks. Pressure, which is usually recorded in kilobars (1000 bars, abbreviated kbar), increases at a rate of 0.3 to 0.4 kbar per kilometer of depth (see Figure 6.1). One bar is approximately equivalent to the pressure of air at Earth’s surface. A diver touring the deeper part of a coral reef at a depth of 10 m would experience an additional bar of pressure.
Minerals that are stable at the lower pressures near Earth’s surface become unstable and recrystallize into new minerals under the increased pressures deep in Earth’s crust. As we will see in Chapter 7, geologists have subjected rocks to extremely high pressures in the laboratory and recorded the pressures required to cause these changes. With these laboratory data in hand, we can examine the mineralogy and texture of metamorphic rock samples and infer what the pressures were in the area where they formed. Thus, metamorphic mineral assemblages can be used as pressure gauges, or geobarometers. Given a specific assemblage of minerals in a metamorphic rock, we can determine the range of pressures, and therefore the depth, at which the rock must have formed.
153
The Role of Fluids
Metamorphic processes can alter a rock’s mineralogy by introducing or removing chemical components that are soluble in heated water. Hydrothermal fluids accelerate metamorphic chemical reactions because they carry dissolved carbon dioxide as well as other chemical substances—such as sodium, potassium, silica, copper, and zinc—that are soluble in hot water under pressure. As hydrothermal solutions percolate up to the shallower parts of the crust, they react with the rocks they penetrate, changing their chemical and mineral compositions and sometimes completely replacing one mineral with another without changing the rock’s texture. This kind of change in a rock’s composition by fluid transport of chemical substances into or out of it is called metasomatism. Many valuable deposits of copper, zinc, lead, and other metallic ores are formed by this kind of chemical substitution, as we saw in Chapter 3.
Where do these chemically reactive fluids originate? Although most rocks appear to be completely dry and to have extremely low porosity, they characteristically contain water in minute pores (the spaces between grains). This water comes not from the pores of sedimentary rocks—from which most of the water is expelled during diagenesis—but rather from chemically bound water in clays. Water forms part of the crystal structure of metamorphic minerals such as micas and amphiboles. The carbon dioxide dissolved in hydrothermal fluids is derived largely from sedimentary carbonates: limestones and dolostones.