Measuring Absolute Time with Isotopic Clocks
The geologic time scale based on stratigraphy and faunal successions is a relative time scale. It tells us whether one formation or fossil assemblage is older than another, but not how long the eras, periods, and epochs were in actual years. Estimates of how long it takes mountains to erode and sediments to accumulate suggested that most geologic periods had lasted for millions of years, but nineteenth-century geologists did not know whether the duration of any specific period was 10 million years, 100 million years, or even longer.
They did know that the geologic time scale was incomplete. The earliest period of geologic history recorded by faunal successions was the Cambrian, when animal life, in the form of shelly fossils, suddenly appeared in the geologic record. Many rock formations were clearly older, however, because they occurred below Cambrian rocks in stratigraphic successions. But these formations contained no recognizable fossils, so there was no way to determine their relative ages. All such rocks were lumped into the general category Precambrian. What fraction of Earth’s history was locked up in these cryptic rocks? How old was the oldest Precambrian rock? How old was Earth itself?
These questions sparked a huge debate in the latter half of the nineteenth century. Physicists and astronomers argued for a maximum age of less than 100 million years, but most geologists regarded this age as much too young, even though they had no precise data to back them up.
208
Discovery of Radioactivity
In 1896, a major advance in physics paved the way for reliable and accurate measurements of absolute ages. Henri Becquerel, a French physicist, discovered radioactivity in uranium. Within three years, the French chemist Marie Sklodowska-Curie discovered and isolated a new and highly radioactive element, radium.
In 1905, the physicist Ernest Rutherford suggested that the absolute age of a rock could be determined by measuring the decay of radioactive elements found in it. He calculated the age of one rock from measurements of its uranium content. This was the start of isotopic dating, the use of naturally occurring radioactive elements to determine the ages of rocks. Isotopic dating methods were refined over the next few years as more radioactive elements were discovered and the processes of radioactive decay became better understood. Within a decade of Rutherford’s first attempt, geologists were able to show that some Precambrian rocks were billions of years old.
In 1956, the geochemist Clair Patterson measured the decay of uranium in meteorites and terrestrial rocks to determine that the solar system—and, by implication, Earth—was formed 4.56 billion years ago. That age has been modified by less than 10 million years since Patterson’s original measurement, so we might say that he completed the discovery of geologic time.
Radioactive Isotopes: The Clocks in Rocks
How do geologists use radioactivity to determine the age of a rock? Recall that the nucleus of an atom consists of protons and neutrons. For a given element, the number of protons is constant, but the number of neutrons can vary among different isotopes of the same element (see Chapter 3). Most isotopes are stable, but the nucleus of a radioactive isotope can spontaneously disintegrate, or decay, emitting particles and transforming the atom into an atom of a different element. We call the original atom the parent and the product of decay its daughter.
One useful element for isotopic dating is rubidium, which has 37 protons and two naturally occurring isotopes: rubidium-85, which has 48 neutrons and is stable, and rubidium-87, which has 50 neutrons and is radioactive. A neutron in the nucleus of a rubidium-87 atom can spontaneously emit an electron, thus changing into a proton, which remains in the nucleus. The parent rubidium atom thus forms a daughter strontium-87 atom, with 38 protons and 49 neutrons (Figure 8.13).
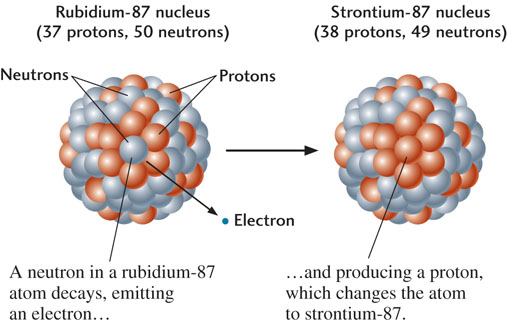
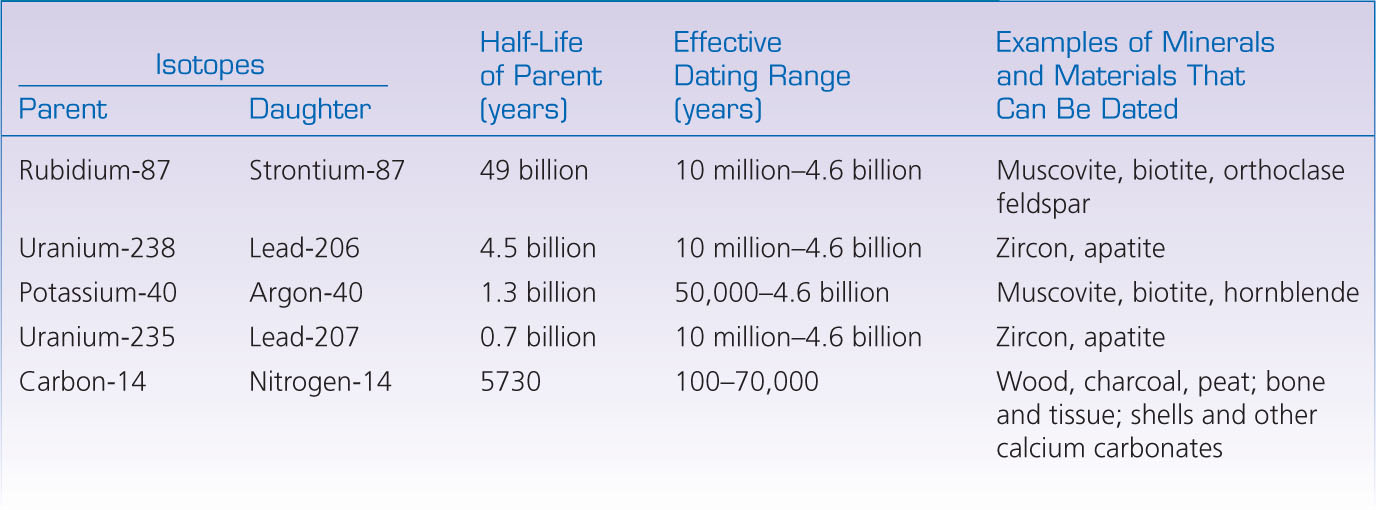
A parent isotope decays into a daughter isotope at a constant rate. The rate of radioactive decay is measured by the isotope’s half-life: the time required for one-half of the original number of parent atoms to be transformed into daughter atoms. At the end of the first half-life, the number of parent atoms has decreased by a factor of two; at the end of the second half-life, by a factor of four; at the end of the third half-life, by a factor of eight, and so forth. As the parent decays, the amount of the daughter isotope increases, preserving the total number of atoms (Figure 8.14). The half-lives of radioactive elements commonly used for isotopic dating are given in Table 8.1.
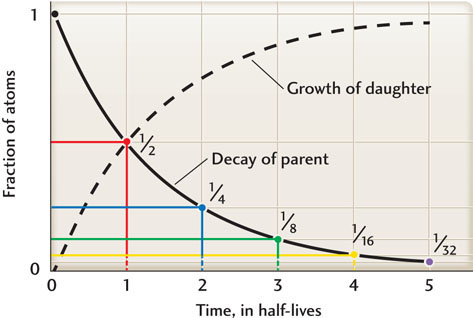
Radioactive isotopes make good clocks because their half-lives do not vary with the changes in temperature, pressure, chemical environment, or other factors that can accompany geologic processes on Earth or other planets. So when atoms of a radioactive isotope are created anywhere in the universe, they start to act like a ticking clock, steadily transforming from one type of atom to another at a fixed rate.
We can measure the ratio of parent to daughter atoms in a rock sample with a mass spectrometer—a precise and sensitive instrument that can detect even minute quantities of isotopes—and determine how much of the daughter has been produced from the parent. Knowing the half-life, we can then calculate the time elapsed since the isotopic clock began to tick.
209
The isotopic age of a rock corresponds to the time since the isotopic clock was “reset” when the isotopes were locked into the minerals of the rock. This “locking” usually occurs when a mineral crystallizes from a magma or recrystallizes during metamorphism. During crystallization, however, the number of daughter atoms in a mineral is not necessarily reset to zero, so the initial number of daughter atoms must be taken into account when calculating isotopic age (see the Practicing Geology exercise at the end of the chapter).
Many other complications make isotopic dating a tricky business. A mineral can lose daughter isotopes by weathering or be contaminated by fluids circulating in the rock. Metamorphism of igneous rocks can reset the isotopic age of minerals in those rocks to a date much later than their crystallization age.
Isotopic Dating Methods
Isotopic dating is possible only if a measurable number of parent and daughter atoms remain in the sample being dated. For example, if a rock is very old and the decay rate of an isotope is fast, almost all the parent atoms will already have been transformed. In that case, we could determine that the isotopic clock had run down, but we would not be able to say when. Thus, isotopes that decay slowly over billions of years, such as rubidium-87, are most useful in measuring the ages of older rocks, whereas those that decay rapidly, such as carbon-14, can only be used to date younger rocks (see Table 8.1).
Carbon-14, which has a half-life of about 5700 years, is especially useful for dating bone, shell, wood, and other organic materials in sediments less than a few tens of thousands of years old. Carbon is an essential element in the living cells of all organisms. As green plants grow, they continuously incorporate carbon into their tissues from carbon dioxide in the atmosphere. When a plant dies, however, it stops absorbing carbon dioxide. At the moment of death, the ratio of carbon-14 to the stable isotope carbon-12 in the plant is identical to that in the atmosphere. Thereafter, the ratio decreases as the carbon-14 in the dead tissue decays. Nitrogen-14, the daughter isotope of carbon-14, is a gas and thus leaks from the material, so it cannot be measured to determine the time that has elapsed since the plant died. We can, however, estimate the absolute age of the plant material by comparing the ratio of carbon-14 left in the plant material with the ratio in the atmosphere at the time the plant died. The latter ratio can be estimated from carbon-14 ages calibrated using other measures of absolute time, such as dendrochronology (counting tree rings).
One of the most precise dating methods for old rocks is based on the decay of two related isotopes: the decay of uranium-238 to lead-206 and the decay of uranium-235 to lead-207. Isotopes of the same element behave similarly in the chemical reactions that alter rocks because the chemistry of an element depends mainly on its atomic number, not its atomic mass. The two uranium isotopes have different half-lives, however, so together they provide a consistency check that helps geologists compensate for the problems of weathering, contamination, and metamorphism discussed above. The lead isotopes from single crystals of zircon—zirconium silicate, a crustal mineral with a relatively high concentration of uranium—can be used to date the oldest rocks on Earth with an uncertainty of less than 1 percent. These formations turn out to be more than 4 billion years old.
210