BOX 5-3 ASTRONOMY DOWN TO EARTH
Photons at the Supermarket
A beam of light can be regarded as a stream of tiny packets of energy called photons. The Planck relationships E = hc/λ and E = hν can be used to relate the energy E carried by a photon to its wavelength λ and frequency ν.
As an example, the laser bar-code scanners used at stores and supermarkets emit orange-red light of wavelength 633 nm. To calculate the energy of a single photon of this light, we must first express the wavelength in meters. A nanometer (nm) is equal to 10−9 m, so the wavelength is
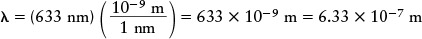
Then, using the Planck formula E = hc/λ, we find that the energy of a single photon is
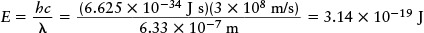
This amount of energy is very small. The laser in a typical bar-code scanner emits 10−3 joule of light energy per second, so the number of photons emitted per second is
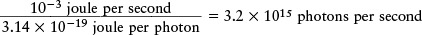
This number is so large that the laser beam seems like a continuous flow of energy rather than a stream of little energy packets.