11-7 The atmospheres of Venus and Mars were very different billions of years ago
The early atmospheres of Earth, Venus, and Mars were predominantly water vapor and carbon dioxide
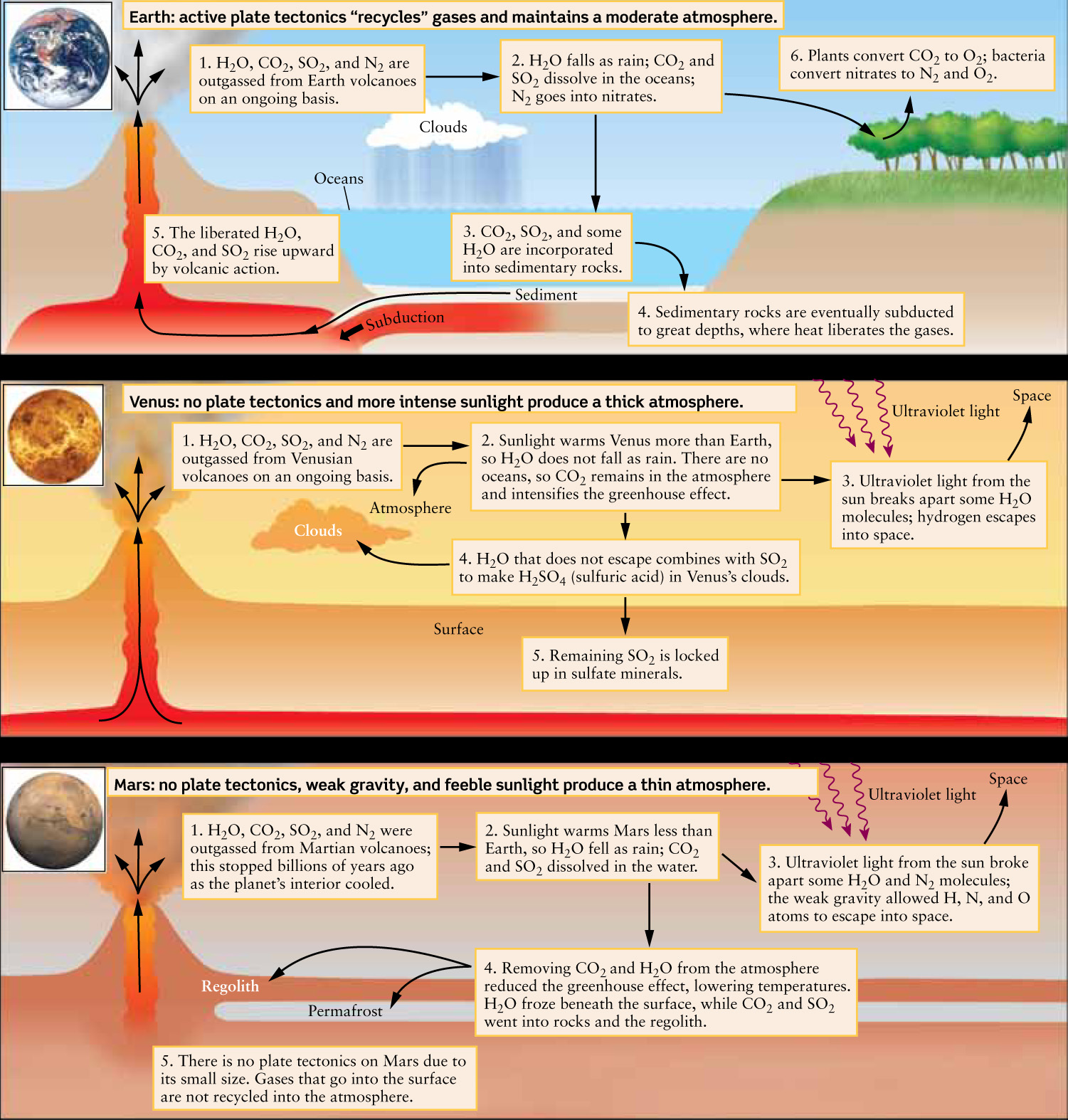
 Why do Earth, Venus, and Mars have such dramatically different atmospheres? As we will see, the original atmospheres of all three worlds were essentially the same. However, each atmosphere evolved in a unique way determined by the planet’s size and distance from the Sun. The Cosmic Connections figure summarizes the processes that led to the present-day atmospheres of the three large terrestrial planets.
Why do Earth, Venus, and Mars have such dramatically different atmospheres? As we will see, the original atmospheres of all three worlds were essentially the same. However, each atmosphere evolved in a unique way determined by the planet’s size and distance from the Sun. The Cosmic Connections figure summarizes the processes that led to the present-day atmospheres of the three large terrestrial planets.
The Origin of Atmospheres on Earth, Venus, and Mars

A Volcanic Eruption on Earth The eruption of Mount St. Helens in Washington State on May 18, 1980, released a plume of ash and gas 40 km (25 mi) high. Eruptions of this kind on Earth, Venus, and Mars probably gave rise to those planets’ original atmospheres.
The original atmospheres of Earth, Venus, and Mars all derived from gases that were emitted, or outgassed, from volcanoes (Figure 11-32). The gases released by present-day Earth volcanoes are predominantly water vapor (H2O), followed by carbon dioxide (CO2), sulfur dioxide (SO2), and nitrogen (N2). These gases should therefore have been important parts of the original atmospheres of Earth, Venus, and Mars. It is also possible—but far from certain—that icy comets from the outer solar system delivered significant quantities of water to these planets.
Due in part to intense volcanic activity that took place when the young planets had warmer interiors, water probably once dominated the atmospheres of Earth, Venus, and Mars. Studies of how stars evolve suggest that the early Sun was only about 70% as luminous as it is now, so the temperature in Venus’s early atmosphere must have been quite a bit lower. Water vapor may actually have been able to liquefy and form oceans on Venus. Mars is smaller than either Earth or Venus, but its large volcanoes could also have emitted substantial amounts of water vapor. With a thicker atmosphere and hence greater pressure, liquid water could also have existed on Mars.
The Evolution of Earth’s Atmosphere
On the present-day Earth most of the water is in the oceans. Nitrogen, which is not very reactive, is still in the atmosphere. By contrast, carbon dioxide dissolves in water, which can fall as rain; as a result, rain removed most of the H2O and CO2 out of our planet’s atmosphere long ago. The dissolved CO2 reacted with rocks in rivers and streams, and the residue was ultimately deposited on the ocean floors, where it turned into carbonate rocks, such as limestone. Consequently, most of Earth’s carbon dioxide is tied up in Earth’s crust, and only about 1 in every 3000 molecules in our atmosphere is a CO2 molecule. If Earth became as hot as Venus, much of its CO2 would be boiled out of the oceans and baked out of the crust, and our planet would soon develop a thick, oppressive carbon dioxide atmosphere much like that of Venus.
The small amount of carbon dioxide in our atmosphere is sustained in part by plate tectonics. Tectonic activity causes carbonate rocks to cycle through volcanoes, where they are heated and forced to liberate their trapped CO2. This liberated gas then rejoins the atmosphere. Without volcanoes, rainfall would remove all of the CO2 from our atmosphere in only a few thousand years. This lack of CO2 would dramatically reduce the greenhouse effect, our planet’s surface temperature would drop precipitously, and the oceans would freeze. Plate tectonics is thus essential for maintaining our planet’s livable temperatures.
The oxygen (O2) in our atmosphere is the result of photosynthesis by plant life, which absorbs CO2 and releases O2. The net result is an atmosphere that is predominantly N2 and O2, with enough pressure and a high enough temperature (thanks to the greenhouse effect) for water to remain a liquid—and hence for life, which requires liquid water, to exist.
COSMIC CONNECTIONS
Evolution of Terrestrial Atmospheres
Earth, Venus, and Mars formed with similar original atmospheres. However, these atmospheres changed dramatically over time due to factors such as planetary size and distance from the Sun.
The Evolution of the Venusian Atmosphere: A Runaway Greenhouse Effect
When Venus was young and had liquid water, the amount of atmospheric CO2 was kept in check much as on Earth today: CO2 was released by volcanoes, then dissolved in the oceans and bound up in carbonate rocks. Enough of the liquid water would have vaporized to create a thick cover of water vapor clouds. Since water vapor is a greenhouse gas, this humid atmosphere—perhaps denser than Earth’s present-day atmosphere, but far less dense than the atmosphere that surrounds Venus today—would have efficiently trapped heat from the Sun. At first, this would have had little effect on the oceans of Venus. Although the temperature would have climbed above 100°C, the boiling point of water at sea level on Earth, the added atmospheric pressure from water vapor would have kept the water in Venus’s oceans in the liquid state.
This hot and humid state of affairs may have persisted for several hundred million years. But as the Sun’s energy output slowly increased over time, the temperature at the surface would eventually have risen above 374°C (647 K, or 705°F). Above this temperature, no matter what the atmospheric pressure, Venus’s oceans would have begun to evaporate. The added water vapor in the atmosphere would have increased the greenhouse effect, making the temperature even higher and causing the oceans to evaporate faster. This temperature increase would have added even more water vapor to the atmosphere, further intensifying the greenhouse effect and making the temperature climb higher still. This is an example of a runaway greenhouse effect, in which an increase in temperature causes a further increase in temperature, and so on.
ANALOGY
A runaway greenhouse effect is like a house in which the thermostat has accidentally been connected backward. Normally, when the temperature rises too high in a house, the heater shuts off. However, if a thermostat is connected backward, the hotter the house, the greater the heater output, raising the temperature even further.
Once Venus’s oceans disappeared, so did the mechanism for removing carbon dioxide from the atmosphere. With no oceans to dissolve it, outgassed CO2 began to accumulate in the atmosphere, making the greenhouse effect “run away” even faster. Temperatures eventually became high enough to “bake out” any CO2 that was trapped in carbonate rocks. This liberated carbon dioxide formed the thick atmosphere of present-day Venus.
Sulfur dioxide (SO2) is also a greenhouse gas released by volcanoes. Although present in far smaller amounts than carbon dioxide, it would have contributed to Venus’s rising temperatures. Like CO2, it dissolves in water, a process that helps moderate the amount of sulfur dioxide in our atmosphere. But when the oceans evaporated on Venus, the amount of atmospheric SO2 would have increased.
The temperature in Venus’s atmosphere would not have increased indefinitely. In time, solar ultraviolet radiation striking molecules of water vapor—the dominant cause of the runaway greenhouse effect—would have broken them into hydrogen and oxygen atoms. The lightweight hydrogen atoms would have then escaped into space (see Box 7-2 for a discussion of why lightweight atoms can escape a planet more easily than heavy atoms). The remaining atoms of oxygen, which is one of the most chemically active elements, would have readily combined with other substances in Venus’s atmosphere. Eventually, almost all of the water vapor would have been irretrievably lost from Venus’s atmosphere. With all the water vapor gone, and essentially all the carbon dioxide removed from surface rocks, the greenhouse effect would no longer have “run away,” and the rising temperature would have leveled off.
Today, the infrared-absorbing properties of CO2 have stabilized Venus’s surface temperature at its present value of 460°C. Only minuscule amounts of water vapor—about 30 parts per million, or 0.003%—remain in the atmosphere. As on Earth, volcanic outgassing might still add small amounts of water vapor to the atmosphere, along with carbon dioxide and sulfur dioxide. But on Venus, these water molecules either combine with sulfur dioxide to form sulfuric acid clouds or break apart due to solar ultraviolet radiation. The great irony is that this state of affairs is the direct result of an earlier Venusian atmosphere that was predominantly water vapor!
The Evolution of the Martian Atmosphere: A Runaway Icehouse Effect
Mars probably had a thicker atmosphere 4 billion years ago. Thanks to Mars’s greater distance from the Sun and hence less intense sunlight, temperatures would have been lower than on young Earth, and any water in the atmosphere would more easily have fallen as rain or snow. This precipitation would have washed much of the planet’s carbon dioxide from its atmosphere, perhaps creating carbonate minerals in which the CO2 is today chemically bound. Measurements from Mars orbit show only small amounts of carbonate materials on the surface, suggesting that the amount of atmospheric CO2 that rained out was small. Hence, even the original Martian atmosphere was relatively thin, though thicker than the present-day atmosphere.
Because Mars is so small, it cooled early in its history and volcanic activity came to an end. Thus, any solid carbonates were not recycled through volcanoes as they are on Earth. The depletion of carbon dioxide from the Martian atmosphere into the surface would therefore have been permanent.
As the amount of atmospheric CO2 declined, the greenhouse effect on Mars would have weakened and temperatures begun to fall. This temperature decrease would have caused more water vapor to condense into rain or snow and fall to the surface, taking even more CO2 with them and further weakening the greenhouse effect. Thus, a decrease in temperature would have caused a further decrease in temperature—a phenomenon sometimes called a runaway icehouse effect. (This is the reverse of the runaway greenhouse effect that has taken place on Venus.) Ultimately, both water vapor and most of the carbon dioxide would have been removed from the Martian atmosphere. With only a very thin CO2 atmosphere remaining, surface temperatures on Mars eventually stabilized at their present frigid values.
As both water vapor and carbon dioxide became depleted, ultraviolet light from the Sun could penetrate the thinning Martian atmosphere to strip it of nitrogen. Nitrogen molecules (N2) normally do not have enough thermal energy to escape from Mars, but they can acquire that energy from ultraviolet photons, which break the molecules in two. Ultraviolet photons can also split carbon dioxide and water molecules, giving their atoms enough energy to escape. Indeed, in 1971 the Soviet Mars 2 spacecraft found a stream of oxygen and hydrogen atoms (from the breakup of water molecules) escaping into space. Oxygen atoms that did not escape into space could have combined with iron-bearing minerals in the surface, forming rustlike compounds. Such compounds have a characteristic reddish-brown color and may be responsible for the overall color of the planet; if this is correct, Mars gained its color by losing its atmosphere!
A Magnetic Field Protects the Martian Atmosphere
Some scientists think a large asteroid impact triggered the loss of the Martian atmosphere. To understand the possible effect of an impact, we need to understand how the Martian atmosphere might have been protected. Ancient magnetic stripes on Mars’s surface indicate that early on, Mars had a magnetic field arising from a magnetic dynamo in a molten core, similar to Earth’s magnetic field (see Section 11-5). As with Earth’s magnetic field, the Martian field would have protected the planet’s atmosphere by deflecting the solar wind away from Mars (see Figure 9-19). The protection arises because if they are not deflected, high-speed particles in the solar wind can break apart atmospheric CO2 and H2O, allowing their lighter constituents to escape into space. Furthermore, the timeline of magnetic signatures in Martian craters suggest that this protective magnetic field persisted for about Mars’s first 500 million years, which coincides with the Late Heavy Bombardment (see Section 8-6).
Putting all of these processes together, a new idea proposes that a very large asteroid impact on Mars disrupted the Martian magnetic dynamo, shut down its magnetic field, and led to the loss of the planet’s atmosphere before its interior cooled down. Advocates of this scenario estimate that there are several large craters of the right age that indicate enough impact energy to significantly disrupt the core convection that produced a magnetic field. However, others are not convinced that such an impact could significantly alter the magnetic field.
Due to Mars’s small size, it only contained enough heat for a short period of volcanism. Therefore, Mars may have been destined to lose its atmosphere anyway. Since planetary cooling shuts down the core convection that creates a global magnetic field (which protects CO2 and H2O), an atmosphere cannot outlast its planet’s internal heat for very long. Whether or not a large impact brought an early end to the Martian atmosphere remains unknown.
Before it was lost, was the Martian atmosphere thick enough and warm enough for water to remain as a liquid on the planet’s surface? As we will see in the next section, scientists have found convincing evidence that the answer is yes.
CONCEPT CHECK 11-9
What is “runaway” about Venus’s runaway greenhouse?
The “runaway” term refers to the fact that an increase in temperature causes changes in the atmosphere, which causes the temperature to rise even higher and higher.
CONCEPT CHECK 11-10
How would a global magnetic field on a young Mars help to keep the planet warm?
A global magnetic field can deflect the solar wind, protecting the Martian atmosphere from high-energy collisions. These collisions can break apart the greenhouse gases CO2 and H2O, allowing the constituent atoms to escape into space.