7-4 The Jovian planets are made of lighter elements than the terrestrial planets
Spectroscopic observations from Earth and spacecraft show that the outer layers of the Jovian planets are composed primarily of the lightest gases, hydrogen and helium (see Box 5-5). In contrast, chemical analysis of soil samples from Venus, Earth, and Mars demonstrate that the terrestrial planets are made mostly of heavier elements, such as iron, oxygen, silicon, magnesium, nickel, and sulfur. Spacecraft images such as Figure 7-5 and Figure 7-6 only hint at these striking differences in chemical composition, which are summarized in Table 7-3.
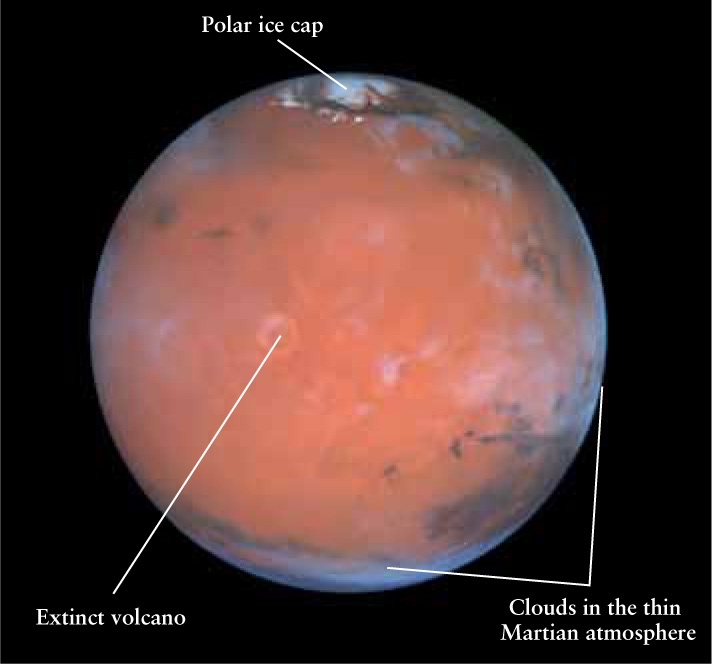
A Terrestrial Planet Mars is composed mostly of heavy elements such as iron, oxygen, silicon, magnesium, nickel, and sulfur. The planet’s red surface can be seen clearly in this Hubble Space Telescope image because the Martian atmosphere is thin and nearly cloudless. Olympus Mons, the extinct volcano to the left of center, is nearly 3 times the height of Mount Everest.
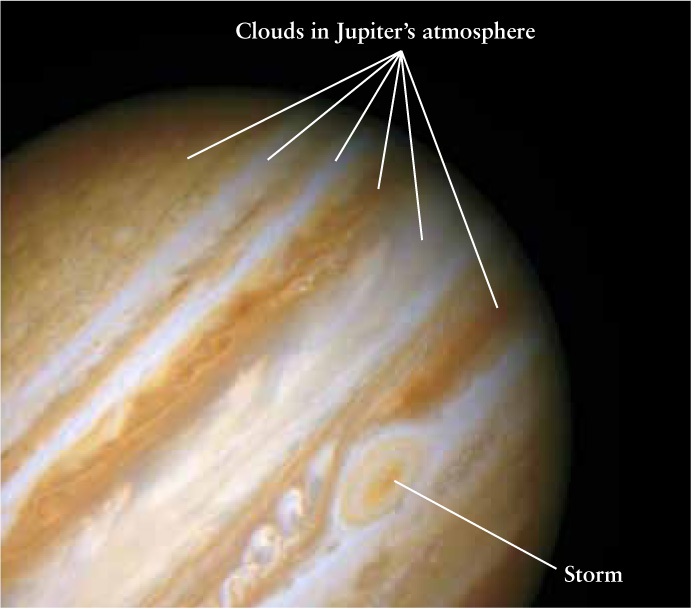
A Jovian Planet This Hubble Space Telescope image gives a detailed view of Jupiter’s cloudtops. Jupiter is composed mostly of the lightest elements, hydrogen and helium, which are colorless; the colors in the atmosphere are caused by trace amounts of other substances. The giant storm at lower right, called the Great Red Spot, has been raging for more than 300 years.
| Terrestrial Planets | Jovian Planets | |
|---|---|---|
| Distance from the Sun | Less than 2 AU | More than 5 AU |
| Size | Small | Large |
| Composition | Mostly rocky materials containing iron, oxygen, silicon, magnesium, nickel, and sulfur | Mostly light elements such as hydrogen and helium |
| Density | High | Low |
Differences in distance from the Sun, and hence in temperature, explain many distinctions between terrestrial and Jovian planets
Temperature plays a major role in determining whether the materials of which planets are made exist as solids, liquids, or gases. Hydrogen (H2) and helium (He) are gaseous except at extremely low temperatures and extraordinarily high pressures. By contrast, rock-forming substances such as iron and silicon are solids except at temperatures well above 1000 K. (You may want to review the discussion of temperature scales in Box 5-1.) Between these two extremes are substances such as water (H2O), carbon dioxide (CO2), methane (CH4), and ammonia (NH3), which solidify at low temperatures (from below 100 to 300 K) into solids called ices. (In astronomy, frozen water is just one kind of “ice.”) At somewhat higher temperatures, they can exist as liquids or gases. For example, clouds of ammonia ice crystals are found in the cold upper atmosphere of Jupiter, but within Jupiter’s warmer interior, ammonia exists primarily as a liquid.
CAUTION!
The Jovian planets are sometimes called “gas giants.” It is true that their primary constituents, including hydrogen, helium, ammonia, and methane, are gases under normal conditions on Earth. But in the interiors of these planets, pressures are so high that these substances are liquids, not gases. The Jovian planets might be better described as “liquid giants”!
As you might expect, a planet’s surface temperature is related to its distance from the Sun. The four inner planets are quite warm. For example, midday temperatures on Mercury may climb to 700 K (= 427°C = 801°F), and during midsummer on Mars, it is sometimes as warm as 290 K (= 17°C = 63°F). The outer planets, which receive much less solar radiation, are cooler. Typical temperatures range from about 125 K (= −148°C = −234°F) in Jupiter’s upper atmosphere to about 55 K (= −218°C = −360°F) at the tops of Neptune’s clouds.
How Temperature Affects Atmospheres
The higher surface temperatures of the terrestrial planets help to explain the following observation: The atmospheres of the terrestrial planets contain virtually no hydrogen molecules or helium atoms. Instead, the atmospheres of Venus, Earth, and Mars are composed of heavier molecules such as nitrogen (N2, 14 times more massive than a hydrogen molecule), oxygen (O2, 16 times more massive), and carbon dioxide (22 times more massive). To understand the connection between surface temperature and the absence of hydrogen and helium, we need to know a few basic facts about gases.
The temperature of a gas is directly related to the speeds at which the atoms or molecules of the gas move: The higher the gas temperature, the greater the speed of its atoms or molecules. Furthermore, for a given temperature, lightweight atoms and molecules move more rapidly than heavy ones. On the four inner, terrestrial planets, where atmospheric temperatures are high, low-mass hydrogen molecules and helium atoms move so swiftly that they can escape from the relatively weak gravity of these planets. Hence, the atmospheres that surround the terrestrial planets are composed primarily of more massive, slower-moving molecules such as CO2, N2, O2, and water vapor (H2O). On the four Jovian planets, low temperatures and relatively strong gravity prevent even lightweight hydrogen and helium gases from escaping into space, and so their atmospheres are much more extensive. The combined mass of Jupiter’s atmosphere, for example, is about a million (106) times greater than that of Earth’s atmosphere. This is comparable to the entire mass of Earth! Box 7-2 describes more about the ability of a planet’s gravity to retain gases.
BOX 7-2 TOOLS OF THE ASTRONOMER’S TRADE
 Kinetic Energy, Temperature, and Whether Planets Have Atmospheres
Kinetic Energy, Temperature, and Whether Planets Have Atmospheres
Amoving object possesses energy as a result of its motion. The faster it moves, the more energy it has (see Figure 4-20). Energy of this type is called kinetic energy. If an object of mass m is moving with a speed ν its kinetic energy Ek is given by

This expression for kinetic energy is valid for all objects, both big and small, from atoms and molecules to planets and stars, as long as their speeds are slow in relation to the speed of light. If the mass is expressed in kilograms and the speed in meters per second, the kinetic energy is expressed in joules (J).
EXAMPLE: An automobile of mass 1000 kg driving at a typical freeway speed of 30 m/s (= 108 km/h = 67 mi/h) has a kinetic energy of
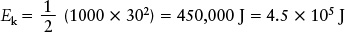
Consider a gas, such as the atmosphere of a star or planet. Some of the gas atoms or molecules will be moving slowly, with little kinetic energy, while others will be moving faster and have more kinetic energy. The temperature of the gas is a direct measure of the average amount of kinetic energy per atom or molecule. The hotter the gas, the faster atoms or molecules move, on average, and the greater the average kinetic energy of an atom or molecule (see Figure 5-11).
If the gas temperature is sufficiently high, typically several thousand kelvins, molecules move so fast that when they collide with one another, the energy of the collision can break the molecules apart into their constituent atoms. Thus, the Sun’s atmosphere, where the temperature is 5800 K, consists primarily of individual hydrogen atoms rather than hydrogen molecules. By contrast, the hydrogen atoms in Earth’s atmosphere (temperature 290 K) are combined with oxygen atoms into molecules of water vapor (H2O).
The physics of gases tells us that in a gas of temperature T (in kelvins), the average kinetic energy of an atom or molecule is
Kinetic energy of a gas atom or molecule
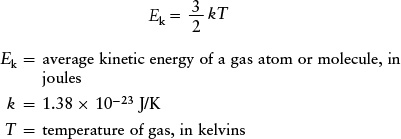
The quantity k is called the Boltzmann constant. Note that the higher the gas temperature, the greater the average kinetic energy of an atom or molecule of the gas. This average kinetic energy becomes zero at absolute zero, or T = 0, the temperature at which molecular motion is at a minimum.
At a given temperature, all kinds of atoms and molecules will have the same average kinetic energy. But the average speed of a given kind of atom or molecule depends on the particle’s mass. To see this dependence on mass, note that the average kinetic energy of a gas atom or molecule can be written in two equivalent ways:
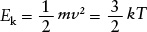
where ν represents the average speed of an atom or molecule in a gas with temperature T. Rearranging this equation, we obtain
Average speed of a gas atom or molecule
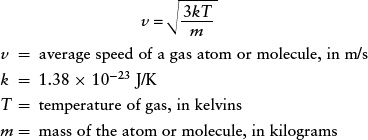
For a given gas temperature, the greater the mass of a given type of gas atom or molecule, the slower its average speed. (The value of v given by this equation is actually slightly higher than the average speed of the atoms or molecules in the gas, but it is close enough for our purposes here. If you are studying physics, you may know that v is actually the root-mean-square speed.)
EXAMPLE: What is the average speed of the oxygen molecules that you breathe at a room temperature of 20°C (= 68°F)?
Situation: We are given the temperature of a gas and are asked to find the average speed of the gas molecules.
Tools: We use the relationship 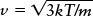 where T is the gas temperature in kelvins and m is the mass of a single oxygen molecule in kilograms.
where T is the gas temperature in kelvins and m is the mass of a single oxygen molecule in kilograms.
Answer: To use the equation to calculate the average speed ν, we must express the temperature T in kelvins (K) rather than degrees Celsius (°C). As we learned in Box 5-1, we do this by adding 273 to the Celsius temperature, so 20°C becomes (20 + 273) = 293 K. The mass m of an oxygen molecule is not given, but you can easily find that the mass of an oxygen atom is 2.66 × 10−26 kg. The mass of an oxygen molecule (O2) is twice the mass of an oxygen atom, or 2(2.66 × 10−26 kg) = 5.32 × 10−26 kg. Thus, the average speed of an oxygen molecule in 20°C air is
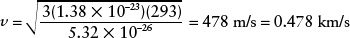
Review: This speed is about 1700 kilometers per hour, or about 1100 miles per hour. Hence, atoms and molecules move rapidly in even a moderate-temperature gas.
In some situations, atoms and molecules in a gas may be moving so fast that they can overcome the attractive force of a planet’s gravity and escape into interplanetary space. The minimum speed that an object at a planet’s surface must have in order to permanently leave the planet is called the planet’s escape speed (see Figure 4-22). The escape speed for a planet of mass M and radius R is given by
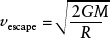
where G = 6.67 × 10−11 N m2/kg2 is the universal constant of gravitation.
The accompanying table gives the escape speed for the Sun, the planets, and the Moon. For example, to escape Earth, a cannon ball would have to be shot with an implausible speed greater than 11.2 km/s (25,100 mi/h).
A good rule of thumb is that a planet can retain a gas if the escape speed is at least 6 times greater than the average speed of the molecules in the gas. (Some molecules are moving slower than average, and others are moving faster, but very few are moving more than 6 times faster than average.) In such a case, very few molecules will be moving fast enough to escape from the planet’s gravity.
EXAMPLE: Consider Earth’s atmosphere. We saw that the average speed of oxygen molecules is 0.478 km/s at room temperature. The escape speed from Earth (11.2 km/s) is much more than 6 times the average speed of the oxygen molecules, so Earth has no trouble keeping oxygen in its atmosphere.
A similar calculation for hydrogen molecules (H2) gives a different result, however. At 293 K, the average speed of a hydrogen molecule is 1.9 km/s. Six times this speed is 11.4 km/s, which is about the escape speed from Earth. Thus, Earth does not retain hydrogen in its atmosphere. Any hydrogen released into the air slowly leaks away into space. On Jupiter, by contrast, the escape speed is so high that even the lightest gases such as hydrogen are retained in its atmosphere. But on Mercury the escape speed is low and the temperature high (so that gas molecules move faster), and Mercury cannot retain any significant atmosphere at all.
| Object | Escape speed (km/s) |
|---|---|
| Sun | 618.0 |
| Mercury | 4.3 |
| Venus | 10.4 |
| Earth | 11.2 |
| Moon | 2.4 |
| Mars | 5.0 |
| Jupiter | 59.5 |
| Saturn | 35.5 |
| Uranus | 21.3 |
| Neptune | 23.5 |
CONCEPT CHECK 7-7
If the average speed of hydrogen molecules in Earth’s atmosphere is below Earth’s escape speed, why do Earth’s atmospheric hydrogen molecules slowly leak into space?
Hydrogen molecules in a gaseous atmosphere have a range of speeds based on the temperature of the gas, with some moving about six times the average speed. These faster molecules exceed Earth’s escape speed and leave Earth entirely as described in Box 7-2. Continuously, some of the remaining slower hydrogen molecules speed up through molecular collisions and steadily escape.