8-2 The cosmic abundances of the chemical elements are the result of how stars evolve
The small sizes of the terrestrial planets compared to the Jovian planets (Property 1 in Table 8-1) suggest that some chemical elements are quite common in our solar system, while others are quite rare. The tremendous masses of the Jovian planets—Jupiter alone has more mass than all of the other planets combined—means that the elements of which they are made, primarily hydrogen and helium, are very abundant. The Sun, too, is made almost entirely of hydrogen and helium. Its average density of 1410 kg/m3 is in the same range as the densities of the Jovian planets (see Table 7-1), and its absorption spectrum (see Figure 5-14) shows the dominance of hydrogen and helium in the Sun’s atmosphere. Hydrogen, the most abundant element, makes up nearly three-quarters of the combined mass of the Sun and planets. Helium is the second most abundant element. Together, hydrogen and helium account for about 98% of the mass of all the material in the solar system. All of the other chemical elements are relatively rare; combined, they make up the remaining 2% (Figure 8-1).
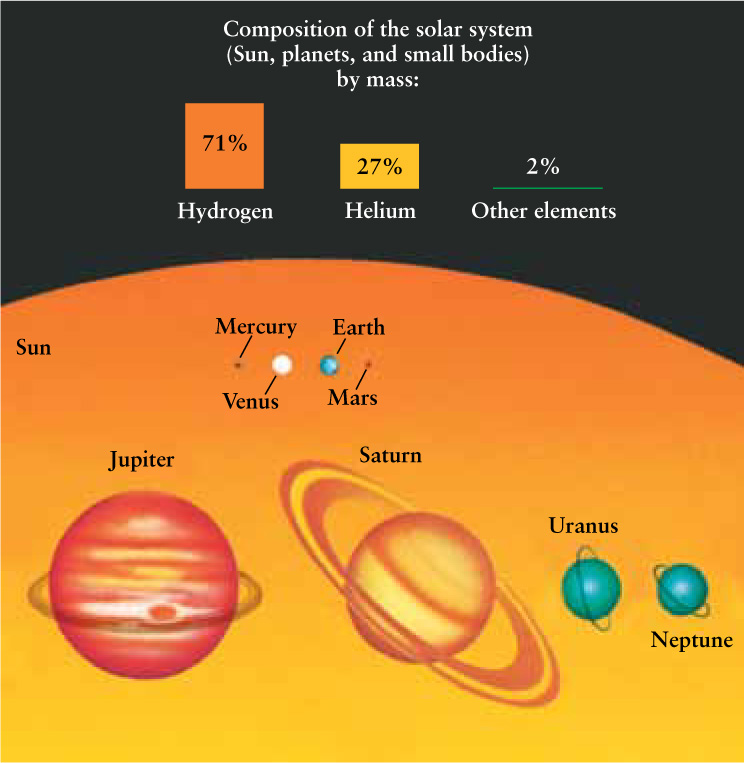
The terrestrial planets are small because they are made of less abundant elements
The dominance of hydrogen and helium is not merely a characteristic of our local part of the universe. By analyzing the spectra of stars and galaxies, astronomers have found essentially the same pattern of chemical abundances out to the farthest distance attainable by the most powerful telescopes. Hence, the vast majority of the atoms in the universe are hydrogen and helium atoms. The elements that make up the bulk of Earth—mostly iron, oxygen, and silicon—are relatively rare in the universe as a whole, as are the elements of which living organisms are made—carbon, oxygen, nitrogen, and phosphorus, among others. (You may find it useful to review the periodic table of the elements, described in Box 5-5.)
The Origin of the Elements and Cosmic “Recycling”
There is a good reason for this overwhelming abundance of hydrogen and helium. A wealth of evidence has led astronomers to conclude that the universe began some 13.7 billion years ago with a violent event called the Big Bang (Chapter 26). Only the lightest elements—hydrogen and helium, as well as tiny amounts of lithium and perhaps beryllium—emerged from the enormously high temperatures following this cosmic event. All the heavier elements were later manufactured by stars, either by thermonuclear fusion reactions deep in their interiors or by the violent explosions that mark the end of massive stars. Were it not for these processes that take place only in stars, there would be no heavy elements in the universe, no planet like our Earth, and no humans to contemplate the nature of the cosmos.
Because our solar system contains heavy elements, it must be that at least some of its material was once inside other stars. But how did this material become available to help build our solar system? The answer is that near the ends of their lives, stars cast much of their matter back out into space. For most stars this process is a comparatively gentle one, in which a star’s outer layers are gradually expelled. Figure 8-2 shows a star losing material in this fashion. This ejected material appears as the cloudy region, or nebulosity (from nubes, Latin for “cloud”), that surrounds the star and is illuminated by it. A few stars eject matter much more dramatically at the very end of their lives, in a spectacular detonation called a supernova, which blows the star apart (see Figure 1-8).

A Mature Star Ejecting Gas and Dust The star Antares is shedding material from its outer layers, forming a thin cloud around the star. We can see the cloud because some of the ejected material has condensed into tiny grains of dust that reflect the star’s light. (Dust particles in the air around you reflect light in the same way, which is why you can see them within a shaft of sunlight in a darkened room). Antares lies some 600 light-years from Earth in the constellation Scorpio.
No matter how it escapes, the ejected material contains heavy elements dredged up from the star’s interior, where they were formed. This material becomes part of the interstellar medium, a tenuous collection of gas and dust that pervades the spaces between the stars. As different stars die, they increasingly enrich the interstellar medium with heavy elements. Observations show that new stars form as condensations in the interstellar medium (Figure 8-3). Thus, these new stars have an adequate supply of heavy elements from which to develop a system of planets, satellites, comets, and asteroids. Our own solar system must have formed from enriched material in just this way. Thus, our solar system contains “recycled” material that was produced long ago inside a now-dead star. This “recycled” material includes all of the carbon in your body, all of the oxygen that you breathe, and all of the iron and silicon in the soil beneath your feet. Scientifically accurate, a rock song from 1970 proclaimed: “We are stardust.”
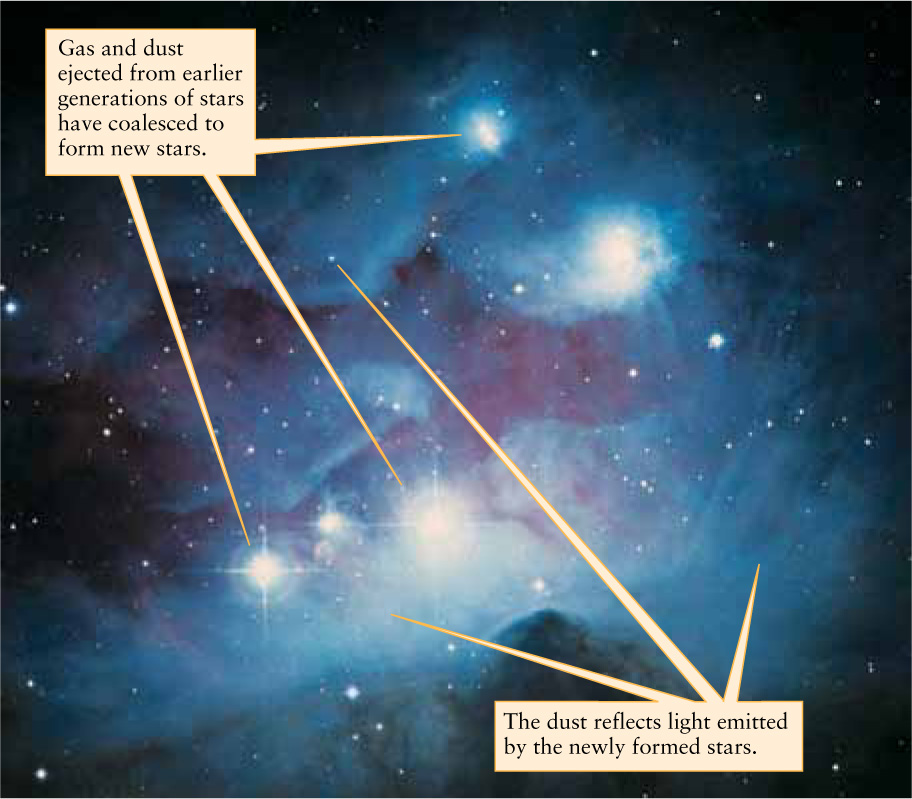
New Stars Forming from Gas and Dust Unlike Figure 8-2, which depicts an old star that is ejecting material into space, this image shows young stars in the constellation Orion (the Hunter) that have only recently formed from a cloud of gas and dust. The bluish, wispy appearance of the cloud (called NGC 1973-1975-1977) is caused by starlight reflecting off interstellar dust grains within the cloud (see Box 5-4). The grains are made of heavy elements produced by earlier generations of stars.
The Abundances of the Elements
Stars create different heavy elements in different amounts. For example, oxygen (as well as carbon, silicon, and iron) is readily produced in the interiors of massive stars, whereas gold (as well as silver, platinum, and uranium) is created only under special circumstances. Consequently, gold is rare in our solar system and in the universe as a whole, while oxygen is relatively abundant (although still much less abundant than hydrogen or helium).
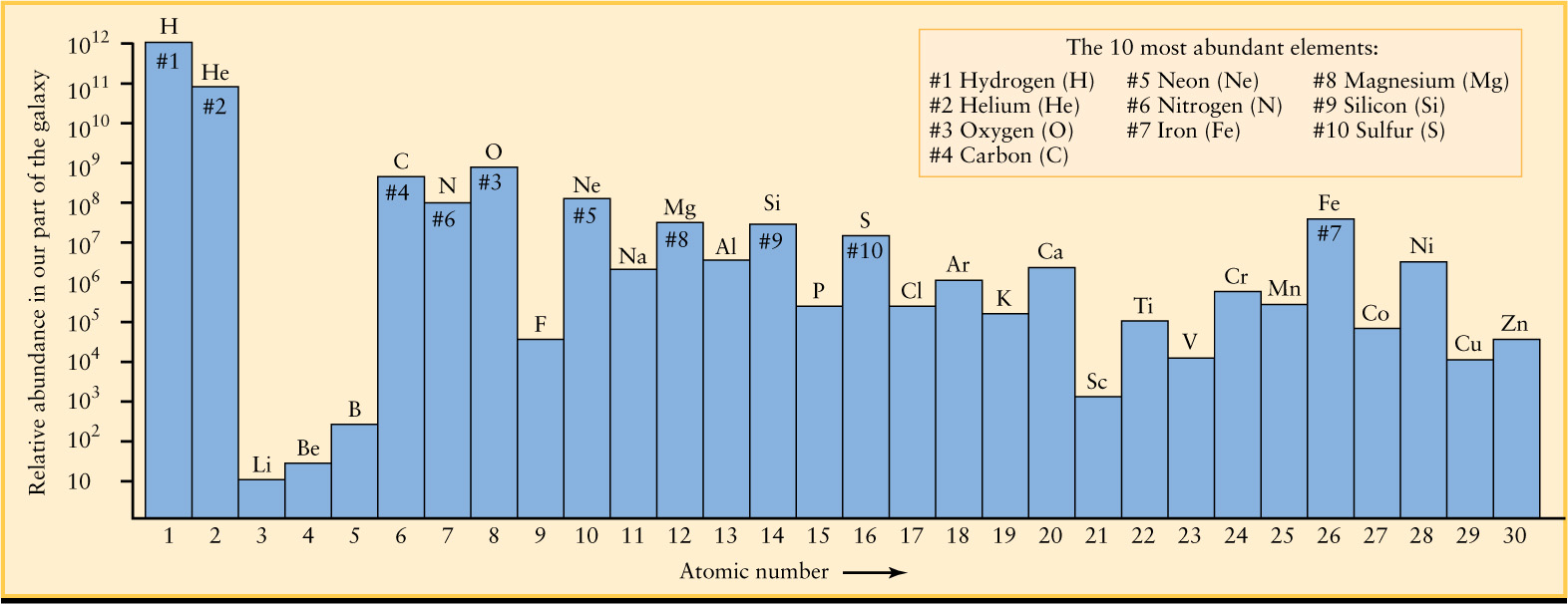
A convenient way to express the relative abundances of the various elements is to say how many atoms of a particular element are found for every trillion (1012) hydrogen atoms. For example, for every 1012 hydrogen atoms in space, there are about 100 billion (1011) helium atoms. From spectral analysis of stars and chemical analysis of Earth rocks, Moon rocks, and bits of interplanetary debris called meteorites, scientists have determined the relative abundances of the elements in our part of the Milky Way Galaxy today. Figure 8-4 shows the relative abundances of the 30 lightest elements, arranged in order of their atomic numbers. An element’s atomic number is the number of protons in the nucleus of an atom of that element. It is also equal to the number of electrons orbiting the nucleus (see Box 5-5). In general, the greater the atomic number of an atom, the greater its mass.
CAUTION!
Figure 8-4 shows that there is about 10 times more hydrogen than helium when comparing the number of atoms. Figure 8-1 shows that our solar system is made of 71% hydrogen versus 27% helium when comparing mass. The explanation of this seeming inconsistency lies in the difference between comparing the mass of atoms versus their numbers: Each helium atom has about 4 times the mass of a hydrogen atom, which makes helium’s contribution to total mass larger than its contribution to the total number of atoms.
The box inset in Figure 8-4 lists the 10 most abundant elements. Note that even oxygen (chemical symbol O), the third most abundant element, is quite rare relative to hydrogen (H) and helium (He): There are only 8.5 × 108 oxygen atoms for each 1012 hydrogen atoms and each 1011 helium atoms. Expressed another way, for each oxygen atom in our region of the Milky Way Galaxy, there are about 1200 hydrogen atoms and 120 helium atoms.
In addition to the 10 most abundant elements listed in Figure 8-4, five elements are moderately abundant: sodium (Na), aluminum (Al), argon (Ar), calcium (Ca), and nickel (Ni). These elements have abundances in the range of 106 to 107 relative to the standard 1012 hydrogen atoms. Most of the other elements are much rarer. For example, for every 1012 hydrogen atoms in the solar system, there are only 6 atoms of gold.
The small cosmic abundances of elements other than hydrogen and helium help to explain why the terrestrial planets are so small (Property 1 in Table 8-1). Because the heavier elements required to make a terrestrial planet are rare, only relatively small planets can form out of them. By contrast, hydrogen and helium are so abundant that it was possible for these elements to form large Jovian planets.
CONCEPT CHECK 8-2
What is meant by the phrase “we are stardust”?
The carbon atoms that make up much of our human bodies and the very oxygen atoms we breathe were made inside of stars. Stars have made most of the atoms other than hydrogen.
CONCEPT CHECK 8-3
From the abundances in Figure 8-4, do you expect that water (H20) might be a common substance in the galaxy?
Yes. Hydrogen (H) is the first most abundant element, and oxygen (O) is the third most abundant element. Water (H20) is, in fact, quite common even though it is usually frozen.