Chemistry lab report, Allyson Goldberg
“Evaluation of the Value of the Gas Constant R” is a lab report written for a chemistry course in which Allyson Goldberg describes and discusses an experiment and its results, following the guidelines in CSE style (see Writing in Action, Chapter 52). You can use the markup tools to highlight and annotate this essay. Try these activities:
- Highlight the in-text references and compare them with citations at the end of the paper to see how the two are connected in CSE style.
- To try a low-stakes practice peer-review session, annotate with questions you would ask the writer. Show your annotations to your instructor for feedback or compare notes with classmates.
Read an annotated version of “Evaluation of the Value of the Gas Constant R” formatted for CSE style
Goldberg 1
Chemistry 119L Laboratory Report
Evaluation of the Value of the Gas Constant R
Allyson Goldberg
Date of Experiment: Monday, September 27, 20XX
Goldberg 2
Introduction
The purpose of this investigation was to experimentally determine the value of the universal gas constant, R. To accomplish this goal, a measured sample of magnesium (Mg) was allowed to react withan excess of hydrochloric acid (HCl) at room temperature and pressure so that the precise amount and volume of the product hydrogen gas (H2) could be determined and the value of R could be calculated using the ideal gas equation, PV=nRT.
Materials & Methods
Two samples of room temperature water, one about 250mL and the other about 400mL, were measured into a smaller and larger beaker respectively. 15.0mL of HCl was then transferred into a side arm flask that was connected to the top of a buret (clamped to a ringstand) through a 5/16” diameter flexible tube. (This “gas buret” was connected to an adjacent “open buret,” clamped to the other side of the ringstand and left open to the atmosphere of the laboratory at its wide end, by a 1/4” diameter flexible tube. These two burets were adjusted on the ringstand so that they were vertically parallel and close together.) The HCl sample was transferred to the flask such that none came in contact with the inner surface of the neck of the flask. The flask was then allowed to rest, in an almost horizontal position, in the smaller beaker. The open buret was adjusted on the ringstand such that its 20mL mark was horizontally aligned with the 35mL mark on the gas buret. Room temperature water was added to the open buret until the water level of the gas buret was at about 34.00mL.
Goldberg 3
A piece of magnesium ribbon was obtained, weighed on an analytical balance, and placed in the neck of the horizontal side arm flask. Next, a screw cap was used to cap the flask and form an airtight seal. This setup was then allowed to sit for 5 minutes in order to reach thermal equilibrium.
After 5 minutes, the open buret was adjusted so that the menisci on both burets were level with each other; the side arm flask was then tilted vertically to let the magnesium ribbon react with the HCl. After the brisk reaction, the flask was placed into the larger beaker and allowed to sit for another 5 minutes.
Next, the flask was placed back into the smaller beaker, and the open buret was adjusted on the ringstand such that its meniscus was level with that of the gas buret. After the system sat for an additional 30 minutes, the open buret was again adjusted so that the menisci on both burets were level.
This procedure was repeated two more times, with the exception that HCl was not again added to the side arm flask, as it was already present in enough excess for all reactions from the first trial.
Results and Calculations
| Trial # | Lab Temp. (ºC) | Lab Pressure (mbar) | Mass of Mg Ribbon Used (g) | Initial Buret Reading (mL) | Final Buret Reading (mL) |
| 1 | 24.4 | 1013 | 0.0147 | 32.66 | 19.60 |
| 2 | 24.3 | 1013 | 0.0155 | 33.59 | N/A* |
| 3 | 25.0 | 1013 | 0.0153 | 34.35 | 19.80 |
*See note in Discussion section.
Goldberg 4
| Trial # | Volume of H2 (L) | Moles of H2 Gas Produced | Lab Temp. (K) | Partial Pressure of H2 (atm) | Value of R (L atm/mol (K) | Mean Value of R (L atm/mol K) |
| 1 | .01306 | 6.05 x 10-4 | 298 | 0.970 | 0.0704 | 0.0728 |
| 2 | N/A | N/A | N/A | N/A | N/A | |
| 3 | 0.01455 | 6.30 x 10-4 | 298 | .968 | 0.0751 |
Table 1 Experimental results
(Sample Calculations—see Table 1)
Volume of H2 gas = final buret reading – initial buret reading
Volume of H2 gas = 32.66mL – 19.60mL = 13.06mL = 0.01306 L
Moles of H2 gas produced = mass of Mg used/molar mass of Mg
Moles of H2 gas produced = 0.0147g/24.305g mol-1 = 6.05 x 10-4 mol
Kelvin temperature = Celsius temperature + 273.15 = 24.3ºC + 273.15
= 298 K
Partial Pressure of H2 gas in the gas buret = Patmosphere – pressure
due to water vapor at the temperature of interest
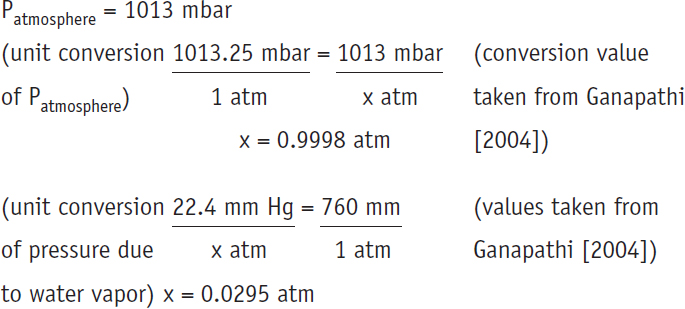
Partial Pressure of H2 gas in the gas buret = 0.9998 atm – 0.0295 atm = 0.9703 atm
Goldberg 5
Value of R:
R = PV/nT
R = [(0.9703 atm) (0.01306 L)]/[(6.05 ´ 10-4 mol) (297.5 K)]
R = 0.01267 L atm/0.180 mol K
R = 0.0704 L atm/mol K
Mean Value of R = (R-value1 + R-value3)/2
Mean Value of R = (0.0704 L atm/mol K + 0.0751 L atm/mol K)/2
Mean Value of R = 0.0728 L atm/mol K
Percent Error = [(measured value - accepted value)/accepted value]
x 100
Percent Error = absolute value of [(0.0728 L atm/mol K – 0.08206 L atm/mol K)/0.08206 L atm/mol K]
x 100
Percent Error = 11.3%
Discussion
Despite the adherence to the experimental procedure, the mean value of R determined in this investigation deviated slightly from the accepted value, 0.08206 L atm/mol K. This deviation was most likely due to the leakage of some H2 gas through the screw cap of the side arm flask during the reaction. Though this could have been better avoided, the tightening of the screw cap onto the side arm flask was necessarily a compromise between an extremely tight seal and a seal too strong to be later removed; thus, any error during experimentation was difficult both to judge and to avoid. In this way, the buret reading after the reaction in Trial 2 was read at 30.80mL. With only a 2.79mL change in the volume of the gas in the buret, it was evident (from comparison with the previous trial) that not all of the hydrogen gas produced in the reaction was captured in the gas buret. Thus, upon recognition of this error, theexperimental procedure for that trial was suspended, and data from Trial 2 was disregarded in analyses.
Goldberg 6
However, detailed attention to pressure was paid during the experiment, such that the position of the open buret was adjusted several times in order to equilibrate its water level with that of the gas buret. This was done to equalize the pressure of the hydrogen gas with that of the atmospheric pressure in the laboratory, as the pressure of the hydrogen gas itself was impossible to measure inside the gas buret. Subsequently, because the atmospheric pressure in the laboratory remained constant throughout the procedure, it was differences in other components of the ideal gas equation (volume, number of moles, and temperature) that made for the varying R-values calculated.
Additionally regarding pressure was the need to account for the contribution of the partial pressure of water vapor when determining the pressure of the H2 gas produced. Because the ideal gas equation was used to calculate R-values, it was necessary to use the purest values possible relating to the hydrogen gas when substituting values into the equation. Though the hydrogen gas produced was not quite an ideal gas, the light weight of and weak attractive forces between its molecules rendered it close enough to one that it was suitable for this experiment. The water vapor molecules, on the other hand, had much larger masses, and the electronegativity of the oxygen atoms added an attractive force that would have added more deviation from the accepted value for R.
Goldberg 7
Accuracy in this experiment could have been compromised by a number of factors. Regarding pressure, more accurate values could have been obtained if the digital barometer used was allowed to have been taken to each workstation, rather than remaining in the front desk; atmospheric pressure likely varied a little bit around the room. Regarding volume, the ruler technique used to confirm that the water level in each buret was horizontally equivalent was helpful, but could have been more effective had the tool used been a level. (Additionally, it was important to note at the beginning of the experiment that both burets were not only close enough together to judge equivalent water levels, but that the two were vertically parallel as well.) Further, the accuracy of temperature measurements could have been improved had there been an apparatus to hold the bulb of the thermometer in midair; resting the thermometer on, or even too near to, the surface of the lab table gave temperature readings that were lower than that of the air surrounding the experimental system.
Conclusions
Conceptually, this experiment was fairly simple to grasp. Experimentally, however, it provided the opportunity to become more intimate with common laboratory equipment, as well as provided a hands-on explanation of the ideal gas equation. For example, the previously learned technique of using a glass stirring rod to channel a liquid into a narrow opening proved to be useful in this procedure when it was necessary to pour HCl into the side arm flask without getting any of the acid inside the neck of the flask.
Goldberg 8
Additionally, the need to account for the partial pressure of water vapor provided a tangible example of what it means to be an ideal gas versus a real gas, and how the concept of the ideal gas equation applies in the real world.
Goldberg 9
References
Ganapathi N. Chemistry 119L laboratory manual. New Haven (CT): Yale University Press; 2004.
Oxtoby DW, Gillis HP, Nachtrieb NH. Principles of modern chemistry. 5th ed. Farmington Hills (MI): Thompson Learning; 2002.