Chapter 2. Laboratory Techniques
Exercise 1. Pipettes and Pipetting
Materials
Flask with water
Rack with eight culture tubes
Blue pi-pump
Green pi-pump
1 mL serological pipette
10 mL serological pipette
Dye solution
Practice cards with circles
p 100 micropipette
PROCEDURE
Pipetting Practice (work independently): Serological Pipettes
Fill four culture tubes with water using serological pipettes.
Tube 1: 8.0 mL water
Tube 2: 1.0 mL water
Tube 3: 0.4 mL water
Tube 4: 0.1 mL water
When you have completed filling each tube compare the tubes from other students at your bench. Are the tubes for the same volume filled to the same level?
When you are finished, empty the tubes and return them to the rack. Place the rack with tubes on the white paper to drain.
Pipetting Practice (work independently): Micropipettes
Using a practice card provided by your lab instructor, pipette 25 microliters (μL), 50 μL, 75 μL, and 100 μL of dye solution in the circles printed on the card.
Exercise 2. Spectrophotometry
Materials
Spectrophotometer
Solutions A and B
Kimwipes
Standard curve
PROCEDURE
Work in groups of two.
- Set the wavelength to 510 nm.
- MODE = Transmittance.
- Set Transmittance to 0% (sample holder is empty).
- Insert blank and set Transmittance to 100%.
- Remove blank and insert sample.
- Switch MODE to Absorbance.
Read Absorbance for the two CoCl2 sample solutions A and B and record these in your lab notebook by creating a data table on an NCR sheet using the following template:
Table 1-1. Absorbance of cobalt chloride solutions.
Concentration Determination
The observations you have made during this exercise measure how much light is absorbed by the solution. These observations must be converted to values that have more relevance (concentration of product) because reaction rates are often expressed as changes in the concentration of product over time. To make these conversions you will use a standard curve. A standard curve is a graph relating a measured quantity to concentration of the substance of interest in “known” samples. The standard curve plots assayed quantity (on the y-axis) vs. concentration (on the x-axis). Such a curve can be used to determine concentrations of the substance in “unknown” samples. Using the standard curve for cobalt chloride shown in Figure 1-5, determine the concentration of CoCl2 for the two sample solutions.
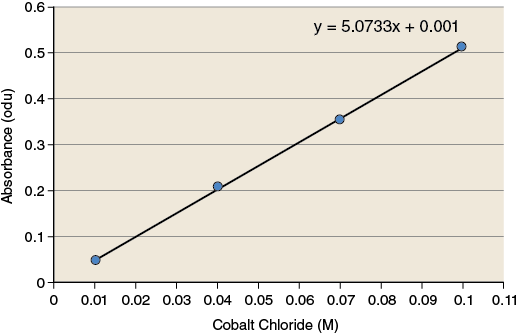
Record the values for the concentration of cobalt chloride for each of the sample solutions in your lab notebook.
Post-Lab Quiz
Proceed to the Post-Lab Quiz