Chapter 2. Carbon Metabolism: Light and Photosynthetic Pigments
General Purpose
This lab will present some of the general concepts related to the process of photosynthesis, including some of the characteristics of light. The technique of paper chromatography will be used as part of the study of the general concepts related to light absorption, transmittance, and fluorescence.
Learning Objectives
General Purpose
- Develop a basic understanding of the reactions and role of photosynthesis.
Conceptual
- Have a general understanding of how a mixture of photosynthetic pigments can be separated from each other by the technique of paper chromatography.
- Know what characteristics of the pigment molecules are important in determining their rates of movement in the technique of paper chromatography.
- Gain an understanding of the relationship between absorbance and transmittance of light and photosynthetic pigments.
- Know what fluorescence is and how it is associated with the absorption of light by chlorophyll.
Procedural
- Gain a proficiency in a chromatographic technique.
- Determine the wavelengths which may be most useful for the process of photosynthesis by determining the absorption spectra of the various pigments separated by paper chromatography.
This laboratory consists of four exercises. You should begin with Exercise 1. Exercises 2 and 3 are demonstrations and Exercise 4 is dependent on you completing Exercise 1.
Exercise 1. Separation and Identification of Chloroplast Pigments by Paper Chromatography
Materials
Chromatography paper: 1 sheet/group (11 cm × 15 cm)
Pigment extract in dark bottle
Chromatography chamber (quart jar)
Chromatography solvent (9 petroleum ether:1 acetone)
Capillary tube
Pencil
Ruler
Forceps
Stapler
Paper towel
Procedure
Work in groups of two and prepare a chromatogram using pigments extracted from either spinach leaves or the cyanobacterium Spirulina.
- Begin this exercise by obtaining a sheet of chromatography paper from your instructor. Handle the paper by the edges only and as little as possible. Oils from your fingers may interfere with the chromatography process. Place the paper on a clean paper towel.
- Use a ruler and make a faint pencil line about 2 cm from and parallel to the long edge of the paper. This line is the origin where the pigments will be applied to the paper.
- Using a capillary tube, apply the pigment extract to the paper along the origin line. The streak should be as thin as possible. Allow the extract to dry for about 30 seconds. Repeat this step about 10 times, keeping the pigment line as narrow as possible. Don’t forget! Allow the extract to dry between applications. When you have completed this step, you should have a dark green band of pigments along the origin line.
- Form a cylinder with the paper and staple the paper at the top and bottom so that the edges do not touch or overlap (Figure 7-7).
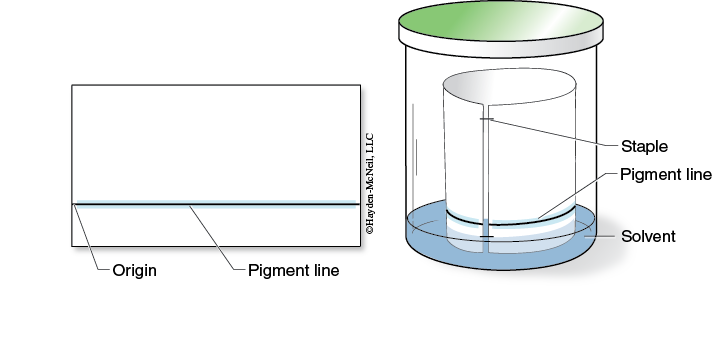
- Obtain a chromatography chamber (quart jar) and add solvent to a depth of about 1 cm or less. The pigment line must be above the solvent level in the chamber. Place the lid on the jar immediately. Wait 5 minutes before you insert the paper cylinder containing the pigment into the chromatography chamber. The chamber should contain a saturated atmosphere of the solvent before the paper is added.
- Use forceps to carefully place the cylinder into the chromatography chamber containing the solvent. Close the lid and allow the pigments to separate.
- When the solvent is within 1 cm of the top of the paper use forceps to remove the paper cylinder from the chromatography chamber. Allow the chromatogram to dry and then remove the staples.
Results
Observe the different pigment bands on the chromatogram. Record your results as a sketch in your laboratory notebook. You might also want to take a picture of the chromatogram if you have a phone with a camera. Record the color of each pigment line and the relative position of the colors along the paper. Gray bands present between the carotene and xanthophylls are phaeophytins, a breakdown product of chlorophyll a and b (chlorophyll a and b without Mg+2).
Interpreting the Results
After you have sketched the chromatogram in your laboratory notebook and identified the various pigments, answer the following questions in your laboratory notebook.
- How does the chromatogram compare with the predictions you made for the pre-lab?
- Which pigment is the most soluble in the chromatography solvent? How do you know?
- Which pigment is the least soluble? How do you know?
- Which pigment appears to be the most nonpolar?
- Which pigment appears to be the most polar?
- What general structural feature of chlorophylls a and b cause them to remain closer to the origin?
CLEAN-UP PROCEDURE
- All organic solutions should be discarded in the container labeled “Pet. Ether and Acetone Waste” near the sink. The solutions should not be poured down the drain. Also, remember to screw the cap back on the container!
- All small pieces of paper should be discarded in the container labeled “Pet. Ether and Acetone Waste.”
- All capillary tubes used in Exercise 1 should be discarded in the “SHARPS” container on your lab bench.
- Dispose of gloves in the biohazard waste container at the front of the classroom.
Exercise 2. Absorption and Transmission of Light
This laboratory exercise is designed to help you better understand some of the principles of the energy-capturing reactions of photosynthesis as demonstrated by the differential absorption and transmission of light by different colored solutions.
Materials
Spectroscope
Colored filters (red, blue, and green)
Pigment extract (from spinach leaves)
Acetone (solvent for the pigment extraction)
PROCEDURE
A spectroscope (Figure 7-8) is a device that contains a prism and displays the total visible spectrum when a white light source is passed through the light slit. If a colored, transparent filter is placed between the light source and the spectroscope, some of the visible rays will be eliminated from the spectrum (as a result of being absorbed by the filter). Therefore, the spectroscope can display the light waves transmitted by the filter.
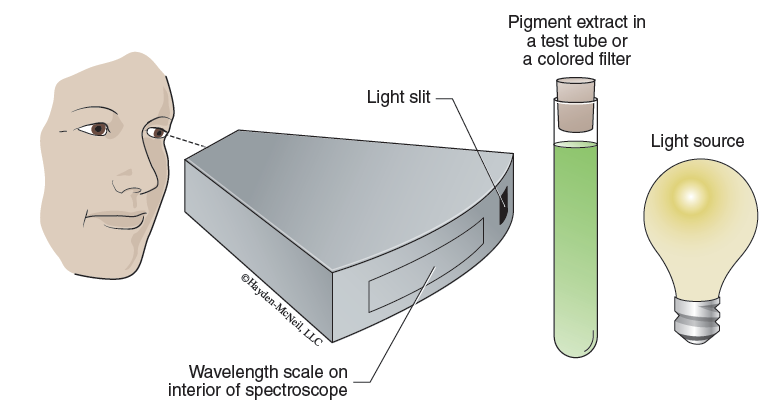
- With the lamp on and positioned such that light is shining through the slit in the back of the spectroscope, observe the visible spectrum through the eyepiece. A nanometer scale will be in view beneath the spectrum. This makes it easy for you to see the different colors of the spectrum and their respective wavelengths in nanometers.
- One by one, place each of the colored filters or solutions (pigment extract and acetone) in front of the light slit of the spectroscope. Observe the spectrum produced by each sample. Make a copy of Table 7-1 in your laboratory notebook and record your results in that table.
Results
Table 7-1. Absorption and transmission of light.
Interpreting the Results
Write out the answers to each of the following questions in your laboratory notebook.
- What is the general relationship between the color of a filter/solution and the light that it absorbs?
- Is there a difference in the wavelength of light absorbed by the green filter and by the pigment extract?
- What is the function of the acetone in this exercise?
Exercise 3. Fluorescence of Spinach Pigment Extract
In their natural setting when chlorophyll molecules have electrons energized by light, the energized electrons are quickly accepted by nearby protein molecules and used in the light reactions. In the case of isolated pigments there aren’t any of these closely associated neighboring protein molecules to accept the energized electrons. One way for those electrons to lose the energy so they return to the normal state is to release the energy as fluorescence.
Materials
Flask of spinach pigment extract
Source of white light (flashlight)
PROCEDURE
In a darkened room, use a flashlight to view the spinach pigment extract.
- View the extract from above while holding the flask so that the light shines through it from below (Figure 7-9A).
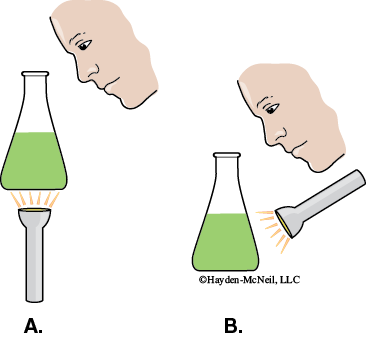
Record the answer to the following question in your laboratory notebook.
What color do you see?
Why?
- View the extract from above while holding the flask such that the light shines on it from above (Figure 7-9B)..
Record the answer to the following question in your laboratory notebook.
What color do you see?
Why?
Exercise 4. Determining the Absorption Spectrum for Spinach Leaf Pigments and Spirulina Pigments
Using a spectrophotometer you can determine the absorbance for each pigment that you separated in Exercise 1 at different wavelengths of light. The instructions for using the spectrophotometer are on the instrument and in Appendix A of this manual. Ask your instructor for assistance if necessary. The instrument should be on. If not, turn the spectrophotometer on and allow it to warm up for 15 minutes.
Materials per Group
5 cuvettes
Parafilm
Acetone
Scissors
Spectrophotometer
4 – 50 mL beakers to elute pigments
Kimwipes
PROCEDURE
- Work in groups of four (or a number assigned by your instructor) for this exercise. Your instructor will assign each group the pigments you separated in Exercise 1.
- Cut out the pigments on your spinach or Spirulina chromatogram and separate them into the
following groups:
Chlorophyll b (absent in Spirulina)
Xanthophylls
Chlorophyll a
Carotene
- Cut up the chromatography paper strips into small beakers labeled chlorophyll b, chlorophyll a, xanthophyll, and carotene. Add 10 mL of acetone to each beaker and swirl gently. Transfer the solution in each beaker to a cuvette, filling the cuvette two-thirds full.
- Fill another cuvette two-thirds full with acetone. This is your blank. The pigment extract was prepared in acetone. The group should now have 5 cuvettes if you are determining the absorbance of spinach pigments; 4 cuvettes if you are determining the absorbance of Spirulina pigments.
- Each group will determine the absorbance for each of their pigments. Create a data table in your lab notebook using Table 7-2 (see Results) as a template.
- Select the beginning wavelength. Begin measurements at 400 nm.
- Zero the instrument by setting it to 0.0% transmittance. A cuvette should not be in the instrument, and the sample holder cover must be closed.
- Insert the blank into the sample holder and close the cover. Remember to align the etched mark on the cuvette with the line on the sample holder. Set transmittance to 100%, then switch the spectrophotometer to absorbance mode. Remove the blank. You are now ready to make your first reading.
- Insert a sample (be sure to align the etched mark) and close the cover. Read and record the absorbance value for each pigment in the data table you created in your laboratory notebook.
- Remove the sample and increase the wavelength by 20 nanometers. Repeat steps 7–9. Continue your observations by taking a reading every 20 nanometers (nm) until you reach 700 nm. You must repeat steps 7–9 every time you change the wavelength.
Note the range of wavelengths for the spectrophotometer you are using. Spectrophotometers are set for 400–580 nm with the filter lever in one position and 600–700 nm with it set in the other position.
Results
In your laboratory notebook record which pigment extract was used for the absorbance measurements.
Table 7-2. Absorbance of different wavelengths of light by photosynthetic pigments.
- Enter your data into an Excel spreadsheet. Create a graph of absorbance vs. wavelength for each isolated pigment. The specific wavelengths of light absorbed by a given pigment form a pattern called its absorption spectrum. An absorption spectrum is somewhat like a “fingerprint” for a pigment.
- Compile the data from all of the groups to create a table in your laboratory notebook using Table 7-3 as a template.
Table 7-3. Wavelengths of greatest absorption for photosynthetic pigments.
CLEAN-UP PROCEDURE
- All organic solutions should be discarded in the container labeled “Pet. Ether and Acetone Waste” near the sink. The solutions should not be poured down the drain. Also, remember to screw the cap back on the container!
- All small pieces of paper should be discarded in the container labeled “Pet. Ether and Acetone Waste.”
- Cuvettes should be rinsed with acetone and turned upside down to drain in the test tube rack near the spectrophotometer you used. A label on the rack indicates the number of cuvettes that the rack should contain. The acetone should be discarded in the container labeled “Pet. Ether and Acetone Waste.”
- Dispose of gloves in the biohazard waste container at the front of the classroom.
Post-Lab Quiz
Proceed to the Post-Lab Quiz