Chapter 2. Carbon Metabolism: Rates of Photosynthesis and Cellular Respiration
General Purpose
This laboratory deals with the general concepts related to the measurement of the rate of photosynthesis indirectly by measuring the pH change generated by net CO2 consumption.
Learning Objectives
General Purpose
Conceptual
- Understand the meaning of net photosynthesis
- Understand the relationship between CO2 level and solution pH
Procedural
- Understand the use of the spectrometer to quantitatively measure the pH of the solutions
Exercise 1. Photosynthetic Rates Under Different Light Colors or Light Intensities (Work in Groups of 2)
Before you begin your experiments write a null and alternative hypothesis about the effects of different light intensities or different wavelengths of light on the net rate of photosynthesis as measured by carbon dioxide consumption in your laboratory notebook.
Write what you predict will happen in this experiment.
Make a note of what the independent variable and the dependent variable are in this experiment.
MATERIALS
4— 50-mL flasks labelled negative control plus a light treatment series (either red, green and blue or high, medium and low)
5 cuvettes
Plant material
Phenol red solution
Straw
PROCEDURE
Part I: Preparing the Phenol Red Solutions
1. Label 4 clean 50-mL flasks either blank, red, blue, and violet or blank, high, medium, and low.
2. With gloves on, use the labelled pipette to dispense 20 mL of phenol red solution into each 50-mL flask.
3. Using a straw, have one person gently blow bubbles into the phenol red solutions while the other person times ten seconds per flask. The phenol red solutions in each flask should turn the same color of yellow/light orange.
Part II: Calibrating the Spectrometer
4.Obtain five cuvettes—one for each treatment and one for a blank and one for a negative control. Rinse the cuvettes if they are not clean.
5. Prepare a blank by filling a clean cuvette halfway with dH2O.
6. Turn on the spectrometer by holding the power button on the right side for about three seconds or until the LED lights start to flash (see Figure 8-10).
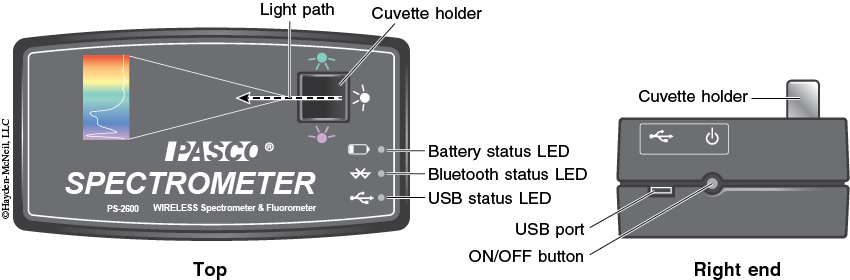
7. Plug the spectrometer into the computer via the USB cable (see Figure 8-10). To wirelessly connect to the spectrometer with a personal laptop or tablet, add a device in the Bluetooth settings and select the spectrometer that matches with the ID # on the bottom of the instrument.
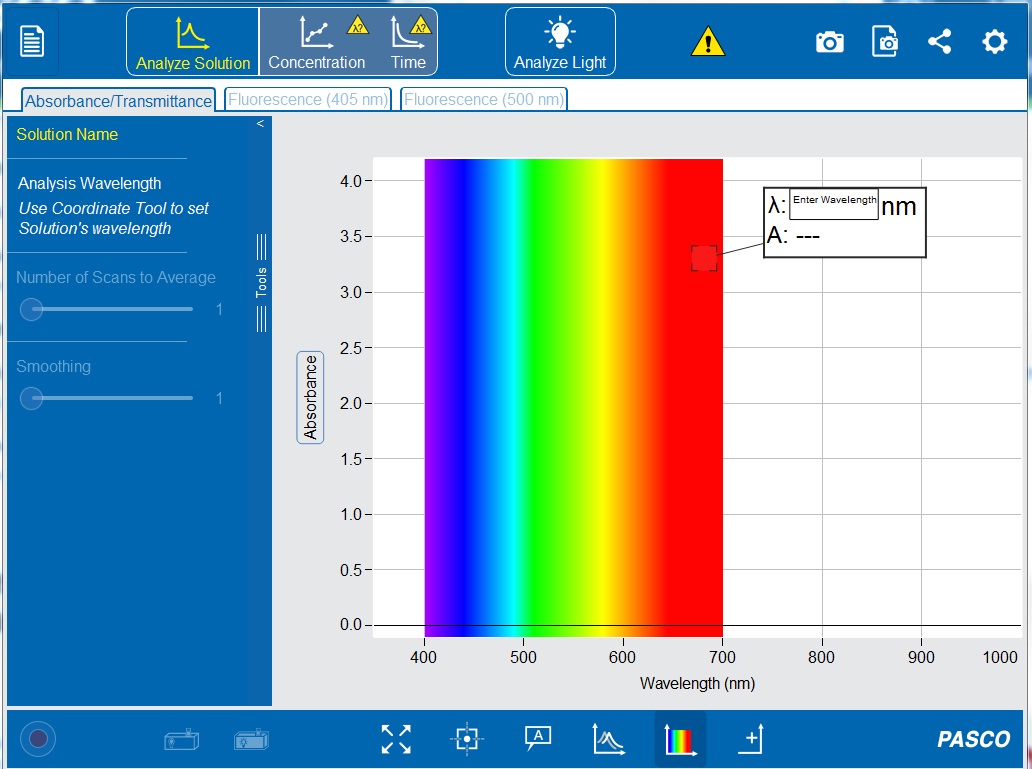
8. Open the PASCO Spectrometry app by clicking the icon ![]() . This should now show the initial screen of the PASCO Spectrometry display (see Figure 8-11) on your computer or tablet.
. This should now show the initial screen of the PASCO Spectrometry display (see Figure 8-11) on your computer or tablet.
9. After a few seconds, the ‘Connection Status’ symbols on the top toolbar should turn from ![]() to
to ![]() . Click the circle to check that the ID # on the bottom of the spectrometer matches the display on your computer or tablet. If connecting wirelessly, a ‘Sensor Not Found’ error should pop up. Click
. Click the circle to check that the ID # on the bottom of the spectrometer matches the display on your computer or tablet. If connecting wirelessly, a ‘Sensor Not Found’ error should pop up. Click ![]() . Select the spectrometer with the corresponding ID # and click
. Select the spectrometer with the corresponding ID # and click ![]() . If a connection error does not pop up, click
. If a connection error does not pop up, click ![]() on the top toolbar.
on the top toolbar.
10. Make sure the “Absorbance/Transmittance” tab ![]() is open on the top toolbar and the “Analyze Solution”
is open on the top toolbar and the “Analyze Solution” ![]() setting is selected.
setting is selected.
11. Wipe the blank cuvette with a Kimwipe and gently insert it into the cuvette holder. The cuvette should fit snugly. Do not remove the parafilm from the top of the cuvettes!
12. On the bottom toolbar, click “Calibrate Dark” ![]() to calibrate the spectrometer with the light turned off. A progress bar should appear, and after the calibration is complete, a check mark
to calibrate the spectrometer with the light turned off. A progress bar should appear, and after the calibration is complete, a check mark ![]() should appear.
should appear.
13. Wait one to two minutes to allow the light source to warm up, then click “Calibrate Reference” ![]() to calibrate the spectrometer to the blank solution with the light on.
to calibrate the spectrometer to the blank solution with the light on.
14. On the bottom toolbar, click “Start Record” ![]() . The symbol will change to the “Stop Record”
. The symbol will change to the “Stop Record” ![]() icon and a horizontal line should appear at 0.0 ODU, indicating the spectrometer was properly calibrated. Tiny peaks in the line show noise, but if there are major peaks, repeat steps 12 and 13 to recalibrate the spectrometer.
icon and a horizontal line should appear at 0.0 ODU, indicating the spectrometer was properly calibrated. Tiny peaks in the line show noise, but if there are major peaks, repeat steps 12 and 13 to recalibrate the spectrometer.
15. In the “Enter Wavelength” box on the display (see Figure 8-11) type 560 nm to set the wavelength to 560 nanometers. If the white Enter Wavelength box is not already displayed on the graph, click ![]() the “Add Coordinate Tool”
on the bottom toolbar. The coordinate tool can also be manually moved to set a wavelength.
the “Add Coordinate Tool”
on the bottom toolbar. The coordinate tool can also be manually moved to set a wavelength.
16. Click ![]() to set the wavelength. A vertical line should appear on the graph at 560 nm, and the white box should now display the wavelength (λ) and the absorbance value (A).
to set the wavelength. A vertical line should appear on the graph at 560 nm, and the white box should now display the wavelength (λ) and the absorbance value (A).
17. Remove the blank cuvette from the spectrometer and return it to the rack.
Part III: Preparing the Cuvettes
18. Use forceps to gently transfer three pre-cut pieces of plant material from the dish on the bench to a dry paper towel. The plant material pieces should be about the same size and have a similar number of leaves.
19. Gently blot off the excess water from the plant material pieces with the paper towel.
20. Use forceps to gently place the blotted pieces of plant material into each flask and push them into the phenol red solutions.
21. Use the labelled 10-mL pipette to transfer ~5 mL of phenol red solution from each flask to a cuvette. Be sure to avoid disturbing the plant material as much as possible. Make note of the initial color in table 8-1 you have prepared in your lab notebook.
Part IV: Manually Measuring Absorbance Over Time
22. For each treatment and for the negative control, wipe the cuvette with a Kimwipe and place it in the cuvette holder. Record absorbance for zero minutes and the initial solution color in Table 8-1 (either a. for varying wavelengths or b. for varying intensities).
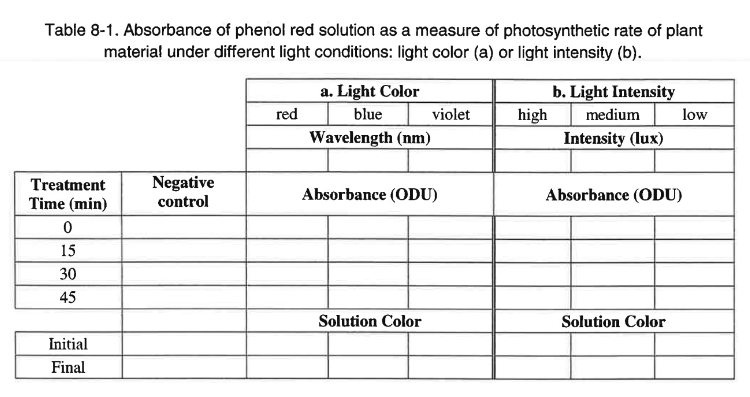
23. Carefully pour the phenol red solution from the cuvettes back into the respective flasks and gently swirl.
24. Place each flask under the appropriate light treatment by group number and set a timer for 15 minutes.
25. After 15 minutes, retrieve the flasks and transfer ~5 mL of phenol red solution from each flask to a cuvette.
26. For each treatment, wipe the cuvette with a Kimwipe, place it in the cuvette holder, and record absorbance in Table 8-1.
27. Return the phenol red solutions to the flasks and gently swirl.
28. Return the flasks to the light treatments and set a timer for 15 minutes.
29. Repeat steps 26-28 until the flasks have been under the light treatments for a total of 45 minutes.
30. Record the final solution color in Table 8-1.
CLEAN-UP PROCEDURE
1. Turn off the spectrometer by holding the power button.
2. Close the PASCO Spectrometry app. Leave the lab computer on.
3. Remove the plant material from the flask to the beaker near the sink. Please do not throw the plant material in the trash or sink.
4. Discard the used phenol red solution in the container by the sink.
5. Rinse all flasks and cuvettes until no phenol red remains. Return them to the racks upside-down to drain.
6. Return the labelled pipettes to the jars.
7. Discard only gloves in the biohazard bag.
8. Return the protocol sheets to the front table.
9. Push in your chair. See your instructor regarding any In-Class Assignment.
Post-Lab Quiz
Proceed to the Post-Lab Quiz