Chapter 2. Spectrophotometry
Spectrophotometry
A spectrophotometer is an instrument designed to detect the amount of radiant energy absorbed by the molecules. To do this, the instrument must have five basic components: a light source, a light dispersion device (either a prism or diffraction grating), an aperture or slit, a detector (a photocell), and a digital meter to display the output of the phototube. The arrangement of these parts is shown in Figure A-1.
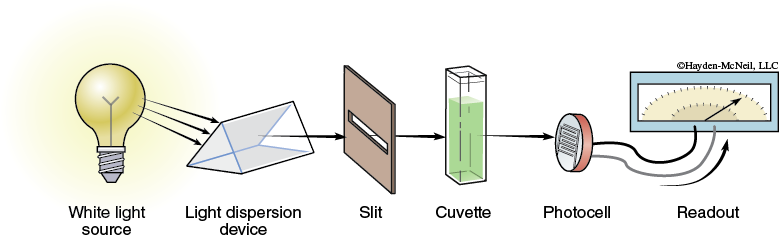
The dispersal device splits white light into its component spectral colors or wavelengths, which then diverge. Various parts of the projecting spectrum can be either blocked or allowed to pass through the slit so that only one wavelength will pass to the rest of the spectrophotometer. (The position of the grating is adjustable so that the region of the spectrum projected on the slit can be changed.) Light that passes through the slit travels through the sample chamber to the photocell, where it creates an electric current proportional to the number of photons striking the phototube. If a digital meter is attached to the phototube, the electric current output can be measured and recorded. The scale is usually calibrated in two ways: percent transmittance (%T), which varies from 0 to 100; and absorbance (A), or optical density units, which runs from 0 to 2. These two factors are related to each other by the following formula:
Before the light-absorbing properties of a solution can be measured, two adjustments on the spectrophotometer are necessary. First, the diffraction grating must be adjusted so that the desired wavelength of light passes through the slit. This is usually the wavelength of light that is best absorbed by the compound under consideration. Secondly, the output of the photocell must be adjusted or calibrated to correct the drift in the electronic circuits and dirt or contaminating material in the light path between the source and the detector. This is done using a “blank” solution. The blank solution should contain everything that the test solution contains except the compound that you are trying to measure.
Because all solutions of chemical compounds absorb light of specific wavelengths, spectrophotometers can be useful in identifying compounds. Furthermore, because the amount of light absorbed is proportional to the concentration of a compound, spectrophotometry is also useful in determining concentrations.
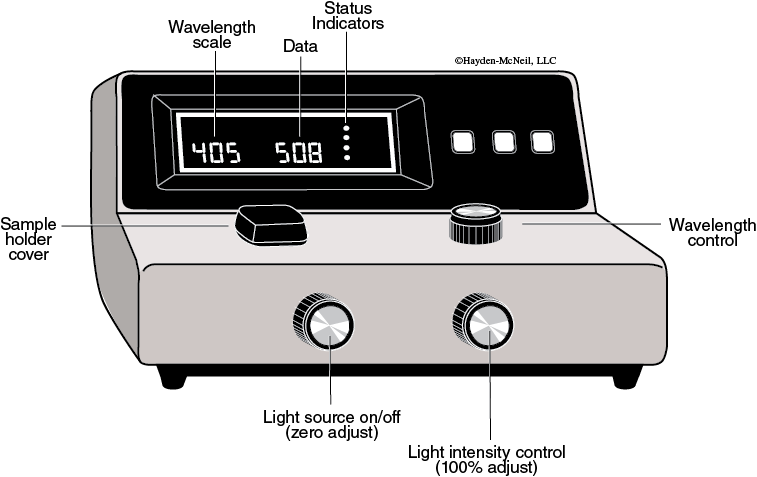
Operating Procedure (Refer to Figure A-2)
- Turn on the spectrophotometer (left-hand knob on the front of the instrument) and allow it to warm up for at least 15 minutes.
- Adjust the wavelength to the appropriate value. The knob on the right top of the instrument controls the wavelength, which is indicated at the left of the digital display.
- With the sample holder empty and the lid closed, adjust the zero adjust knob (left-hand knob) until the instrument reads 0% on the transmittance scale. Be sure that the display function is set to transmittance; if not, push the “Mode” button until the display is set to transmittance.
- Carefully insert the appropriate tube (cuvette) containing the blank solution into the sample holder and close the cover. The cuvette’s outside surface must be dry and clean, which includes being free of fingerprints! Use a Kimwipe to clean the cuvette before inserting. The white markings should line up with the notch on the sample holder. It is important to line up the markings. The cuvette will be scratched otherwise.
- Adjust the 100% adjust knob, on the right front of the instrument, until the display reads 100% on the transmittance scale.
- Remove the blank cuvette and immediately insert the sample cuvette as described in step 4 above. Do not change any instrument setting! Switch the display to read absorbance by pushing the “Mode” button.
- Record the value indicated on the absorbance scale.
- Repeat this procedure for additional cuvettes or wavelengths as required. Always adjust the blank transmittance to 100% before inserting and reading a new set of cuvettes.
Below is a video on how a spec works:
Below is a video on how to use a spectrophotometer.