Chapter 2. Population Ecology
Learning Objectives
General Purpose
Conceptual
- Gain an understanding of the factors that impact populations.
- Gain an understanding of the factors that impact and cause change in ecosystems.
- Gain an understanding of the basic characteristics of the model organism Chlamydomonas reinhardtii.
- Understand the relationship between light absorption and the concentration of a substance.
- Understand how to use a standard curve.
Procedural
- Gain experience setting up controlled population ecology experiments.
- Gain experience graphing.
- Gain proficiency in the use of a spectrophotometer.
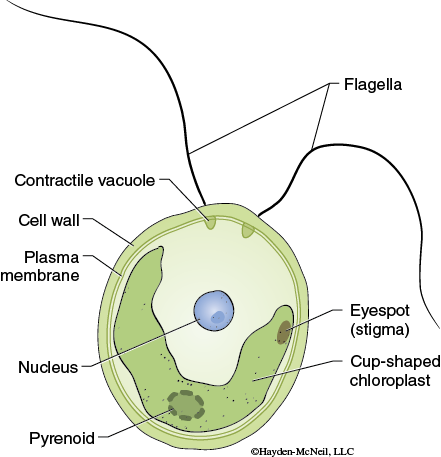
To examine population growth, a model organism is frequently used. The unicellular green alga Chlamydomonas reinhardtii (see Figure 4-9) is the model organism you will use.
The size of a population and whether it is increasing or decreasing have a large impact on these processes. Population growth is often shown as logistic growth (see Figure 4-10).
Logistic growth occurs when exponential growth is limited by the carrying capacity of the ecosystem. In this lab you will examine the impact of a factor on the growth of a population of C. reinhardtii. During the growth of your C. reinhardtii cultures you will gather absorbance data for the replicates of the different treatments in your experiment. Since C. reinhardtii are motile and will swim towards the light in the spectrophotometer, you must take your absorbance readings quickly. The absorbance of your C. reinhardtii cultures will increase as individuals gather near the light slit. Using the standard curve shown below (see Figure 4-11), or the equation, you can convert your absorbance readings to number of cells per mL.
This standard curve shows a linear relationship between population density and absorbance at 750 nm. The equation for the best-fit line for this data is Absorbance = 1.058 (millions of cells/mL) − 0.0022 and the correlation coefficient (R2) is 0.9979.
2.1 Procedure
- Choose which environmental factor your group is going to examine for an impact on the population growth. Select either an abiotic factor from Table 4-3 or a biotic factor from Table 4-4.
Table 4-3. Abiotic factors.
Table 4-4. Biotic factors.
- In your laboratory notebook record which factor you selected and write out your null and alternative hypotheses.
- Your population growth experiment will go on for an extended time during which you will take absorbance readings at predetermined time intervals (ask your instructor what time intervals you will use).
- Choose the procedure your group will use from among the following to determine your specific protocol.
Possible Treatments
Nitrogen
- Collect NINE sterile culture tubes.
- Choose two nitrogen conditions, No nitrogen (No N; Min medium without nitrogen), Low nitrogen (low N; Min medium with reduced nitrogen), High nitrogen (high N; Min medium with extra nitrogen).
- Label all the tubes using a permanent marker in the top one third of the tube. All tubes should have your initials, section number, and replicate information (one each: ctrl-1, ctrl-2, ctrl-3, and choose two of the following: no N-1, no N-2, no N-3; low-1, low-2, low-3; or high-1, high-2, high-3).
- Pipette ONE mL of Chlamydomonas for Nitrogen experiment culture into all tubes. Use 1 mL micropipette with blue pipette tips.
- Pipette 4 mL of Min (minimal media, a mixture of nitrogen, phosphates, and trace metals) into the control tubes, ctrl-1, ctrl-2, ctrl-3. Use 10 mL pipette with the green pi-pump.
- Pipette 4 mL of first nitrogen condition solution into 3 tubes.
- Pipette 4 mL of second nitrogen condition solution into 3 tubes.
- Seal all tubes with Parafilm squares.
- Invert the tubes several times to mix the contents thoroughly.
- Measure the density of your Chlamydomonas population by reading the absorbance at 750 nm for all tubes. MIX THE CONTENTS OF EACH TUBE IMMEDIATELY BEFORE PLACING IT IN THE SPECTROPHOTOMETER. Record the absorbance readings in your laboratory notebook.
- Recheck a blank in the spectrophotometer to assure machine is still blanked correctly.
- Remove Parafilm from sample tubes.
- Place all tubes in rack to be placed under fluorescent lights at room temperature. Recover rack loosely with the plastic wrap provided. Record rack and space number.
- Calculate the density of Chlamydomonas in the tubes using the standard curve (available in moodle).
- Copy this procedure into your lab notebook.
Temperature
- Collect NINE sterile culture tubes.
- Label all the tubes using a permanent marker in the top one third of the tube. All tubes should have your initials, section number, and replicate information (one each: ctrl-1, ctrl-2, ctrl-3, low-1, low-2, low-3, high-1, high-2, high-3).
- Pipette ONE mL of Chlamydomonas culture into all tubes. Use 1 mL micropipette with blue pipette tips.
- Pipette 4 mL of Min (minimal media, a mixture of nitrates, phosphates, and trace metals) into all tubes. Use 10 mL pipette with the green pi-pump.
- Seal all tubes with Parafilm squares.
- Invert the tubes several times to mix the contents thoroughly.
- Measure the density of your Chlamydomonas population by reading the absorbance at 750 nm for all tubes. MIX THE CONTENTS OF EACH TUBE IMMEDIATELY BEFORE PLACING IT IN THE SPECTROPHOTOMETER. Record the absorbance readings in your laboratory notebook.
- Recheck a blank in the spectrophotometer to assure machine is still blanked correctly.
- Remove Parafilm from sample tubes.
- Place the control tubes under fluorescent lights at room temperature. Place the low tubes under fluorescent lights in the refrigerator in the prep room. Place the high tubes under fluorescent lights in the incubator. Place control tubes in control rack. Replace plastic wrap loosely over rack. Record rack and space number. Record temperatures from thermometers.
- Calculate the density of Chlamydomonas in the tubes using the standard curve.
- Copy this procedure into your laboratory notebook.
Gas Exchange
- Collect NINE sterile culture tubes.
- Label all the tubes using a permanent marker in the top one third of the tube. All tubes should have your initials, section number, and replicate information (one each: A-1, A-2, A-3, B-1, B-2, B-3, C-1, C-2, C-3).
- Pipette ONE mL of Chlamydomonas culture into all tubes. Use 1 mL micropipette with blue pipette tips.
- Pipette 4 mL of Min (minimal media, a mixture of nitrates, phosphates, and trace metals) into all tubes. Use 10 mL pipette with the green pi-pump.
- Seal all tubes with Parafilm squares.
- Invert the tubes several times to mix the contents thoroughly.
- Measure the density of your Chlamydomonas population by reading the absorbance at 750 nm for all tubes. MIX THE CONTENTS OF EACH TUBE IMMEDIATELY BEFORE PLACING IT IN THE SPECTROPHOTOMETER. Record the absorbance readings in your laboratory notebook.
- Recheck blank in the spectrophotometer to assure machine is still blanked correctly.
- Remove Parafilm from A-1, A-2, A-3. Poke a small hole in the Parafilm with a dissecting pin in tubes B-1, B-2, B-3. Make sure Parafilm is tightly wrapped around the tube for tubes C-1, C-2, C-3. Have your TA check your tubes.
- Place the control tubes under fluorescent lights at room temperature. Replace plastic wrap loosely over rack. Record rack and space numbers.
- Calculate the density of Chlamydomonas in the tubes using the standard curve (available in moodle).
- Copy this procedure into your lab notebook.
Phosphate
- Collect NINE sterile culture tubes.
- Choose two phosphate conditions, No phosphate (no P; Min medium without phosphate), Low phosphate (low P; Min medium with reduced phosphate), High phosphate (high P; Min medium with extra phosphate).
- Label all the tubes using a permanent marker in the top one third of the tube. All tubes should have your initials, section number, and replicate information (one each: ctrl-1, ctrl-2, ctrl-3, and 2 of the following: no P-1, no P-2, no P-3; low-1, low-2, low-3; or high-1, high-2, high-3).
- Pipette ONE mL of Chlamydomonas for phosphate experiment culture into all tubes. Use 1 mL micropipette with blue pipette tips.
- Pipette 4 mL of Min (minimal media, a mixture of nitrates, phosphates, and trace metals) into control. Use 10 mL pipette with the green pi-pump.
- Pipette 4 mL of first phosphate condition solution into 3 tubes. Use 10 mL pipette.
- Pipette 4 mL of second phosphate condition solution into 3 tubes. Use 10 mL pipette.
- Seal all tubes with Parafilm squares.
- Invert the tubes several times to mix the contents thoroughly.
- Measure the density of your Chlamydomonas population by reading the absorbance at 750 nm for all tubes. MIX THE CONTENTS OF EACH TUBE IMMEDIATELY BEFORE PLACING IT IN THE SPECTROPHOTOMETER. Record the absorbance readings in your laboratory notebook.
- Recheck a blank in the spectrophotometer to assure machine is still blanked correctly.
- Remove Parafilm from sample tubes.
- Place all tubes in rack to be placed under fluorescent lights at room temperature. Recover rack loosely with the plastic wrap provided. Record rack and space number.
- Calculate the density of Chlamydomonas in the tubes using the standard curve (available in moodle).
- Copy this procedure into your lab notebook.
pH
- Collect NINE sterile culture tubes.
- Label all the tubes using a permanent marker in the top one third of the tube. All tubes should have your initials, section number, and replicate information (triplicates of each pH, for example 7-1, 7-2, 7-3).
- Pipette ONE mL of Chlamydomonas culture into all tubes. Use 1 mL micropipette with blue pipette tips.
- Pipette 4 mL of Min (minimal media, a mixture of nitrates, phosphates and trace metals, pH ______). Use 10 mL pipette with the green pi-pump.
- Pipette 4 mL of Min pH ______ (minimal media adjusted to pH ______) into three pH ______tubes.
- Pipette 4 mL of Min pH ______ (minimal media adjusted to pH ______) into three pH ______ tubes.
- Seal all tubes with Parafilm squares.
- Invert the tubes several times to mix the contents thoroughly.
- Measure the density of your Chlamydomonas population by reading the absorbance at 750 nm for all tubes. MIX THE CONTENTS OF EACH TUBE IMMEDIATELY BEFORE PLACING IT IN THE SPECTROPHOTOMETER. Record the absorbance readings in your laboratory notebook.
- Recheck a blank in the spectrophotometer to assure machine is still blanked correctly.
- Remove Parafilm from sample tubes.
- Place all tubes in rack to be placed under fluorescent lights at room temperature. Recover rack loosely with the plastic wrap provided. Record rack and space number.
- Calculate the density of Chlamydomonas in the tubes using the standard curve (available in moodle).
- Copy this procedure into your lab notebook.
Light Intensity
- Collect NINE sterile culture tubes.
- Label all the tubes using a permanent marker in the top one third of the tube. All tubes should have your initials, section number, and replicate information (one each: ctrl-1, ctrl-2, ctrl-3, H-1, H-2, H-3, L-1, L-2, L-3).
- Pipette ONE mL of Chlamydomonas culture into all tubes. Use 1 mL micropipette with blue pipette tips.
- Pipette 4 mL of Min (minimal media, a mixture of nitrates, phosphates, and trace metals) into all tubes. Use 10 mL pipette with the green pi-pump.
- Seal all tubes with Parafilm squares.
- Invert the tubes several times to mix the contents thoroughly.
- Measure the density of your Chlamydomonas population by reading the absorbance at 750 nm for all tubes. MIX THE CONTENTS OF EACH TUBE IMMEDIATELY BEFORE PLACING IT IN THE SPECTROPHOTOMETER. Record the absorbance readings in your laboratory notebook.
- Recheck a blank in the spectrophotometer to assure machine is still blanked correctly.
- Remove Parafilm from sample tubes.
- Place the control tubes under fluorescent lights at room temperature. Place the high light tubes (H-1, H-2, H-3) in the designated area with extra lights. Place the low light tubes (L-1, L-2, L-3) in the designated low light area. Replace plastic wrap loosely over rack. Record rack and space numbers. Record light intensity.
- Calculate the density of Chlamydomonas in the tubes using the standard curve (available in moodle).
- Copy this procedure into your lab notebook.
Intraspecies Competition
- Collect NINE sterile culture tubes.
- Label all the tubes using a permanent marker in the top one third of the tube. All tubes should have your initials, section number, and replicate information (one each: ctrl-1, ctrl-2, ctrl-3, low-1, low-2, low-3, high-1, high-2, high-3).
- Pipette 1 mL of Chlamydomonas culture into control tubes. Pipette 0.5 mL of Chlamydomonas culture into low tubes. Pipette 3 mL of Chlamydomonas culture into high tubes. Use 1 mL micropipette with blue pipette tips. You will have to pipette three times for 3 mL.
- Pipette 4 mL of Min (minimal media, a mixture of nitrates, phosphates, and trace metals) into control tubes. Pipette 4.5 mL of Min into low tubes. Add 2 mL of Min to high tubes. Use 10 mL pipette with the green pi-pump.
- Seal all tubes with Parafilm squares.
- Invert the tubes several times to mix the contents thoroughly.
- Measure the density of your Chlamydomonas population by reading the absorbance at 750 nm for all tubes. MIX THE CONTENTS OF EACH TUBE IMMEDIATELY BEFORE PLACING IT IN THE SPECTROPHOTOMETER. Record the absorbance readings in your laboratory notebook.
- Recheck blank in the spectrophotometer to assure machine is still blanked correctly.
- Remove Parafilm from sample tubes.
- Place all the tubes in rack to be placed under fluorescent lights at room temperature. Recover rack loosely with the plastic wrap provided. Record rack and space number.
- Calculate the density of Chlamydomonas in the tubes using the standard curve.
- Copy this procedure into your laboratory notebook.
Interspecies Competition
Chlamydomonas in the presence of blue-green algae (choose one).
Blue-green algae are bacteria, specifically cyanobacteria.
- Collect NINE sterile culture tubes.
- Label all the tubes using a permanent marker in the top one third of the tube. All tubes should have your initials, section number, and replicate information (one each: Ch-1, Ch-2, Ch-3, both-1, both-2, both-3, BGA-1, BGA-2, BGA-3).
- Pipette 1 mL of Chlamydomonas culture into Chlamydomonas tubes and both tubes. Pipette 1 mL of blue-green algae culture (either Oscillatoria or Anabaena) into blue-green algae tubes and both tubes. Use 1 mL micropipette with blue pipette tips.
- Pipette 4 mL of Min (minimal media, a mixture of nitrates, phosphates, and trace metals) into Chlamydomonas and blue-green algae tubes. Pipette 3 mL of Min into “both” tubes. Use 10 mL pipette with the green pi-pump.
- Seal all tubes with Parafilm squares.
- Invert the tubes several times to mix the contents thoroughly.
- Measure the density of your Chlamydomonas population by reading the absorbance at 750 nm for all tubes. MIX THE CONTENTS OF EACH TUBE IMMEDIATELY BEFORE PLACING IT IN THE SPECTROPHOTOMETER. Record the absorbance readings in your laboratory notebook.
- Recheck a blank in the spectrophotometer to assure machine is still blanked correctly.
- Remove Parafilm from sample tubes.
- Place all the tubes in rack to be placed under fluorescent lights at room temperature. Recover rack loosely with the plastic wrap provided. Record rack and space number.
- Calculate the density of Chlamydomonas in the tubes using the standard curve.
- Copy this procedure into your laboratory notebook.
Once you have selected your specific protocol, make a table in your laboratory notebook in a table similar to the example shown in Table 4-5 to record your absorbance data.
Table 4-5. Absorbance reading from Chlamydomonas cultures.