Chapter 2. ECOSYSTEM ECOLOGY— AQUATIC II—WATER ANALYSIS
Learning Objectives
General Purpose
Conceptual
- Be able to determine the change in an ecosystem caused by either biotic or abiotic factors.
- Be able to determine the impact that nutrient cycles have on ecosystems.
Procedural
- Be able to determine levels of various nutrients in aquatic ecosystems.
- Be able to properly graph data.
- Be able to properly communicate results to a scientific audience in both written and oral form.
General Purpose
Energy flows into and out of ecosystems, whereas nutrients often cycle between several different forms within the ecosystem. Understanding this cycling of nutrients is fundamental to a full understanding of ecosystem ecology. Some forms of nutrients are available to the organisms of the ecosystem and other forms cannot be used directly by many of the organisms in the ecosystem. These unavailable nutrients can act as a reservoir that under the correct circumstances can become available to the organisms of the ecosystem. Since the nutrients in their different forms can be interconverted, it is important to be able to assess the levels of the differing forms of a nutrient within an ecosystem to determine the potential impact on the ecosystem.
The focus of the exercises in this lab is the chemical reactions required for analysis of abiotic factors in samples taken from sites in the University Lake system (see Figure 6-3).
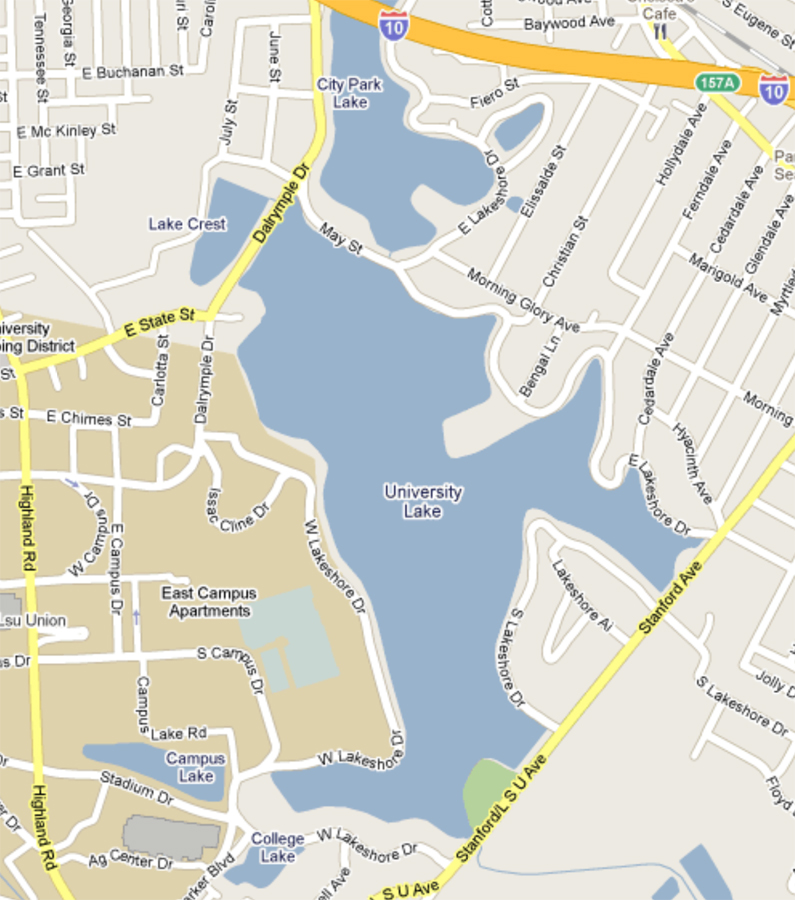
Working in groups your lab will analyze samples from four sites. Data collected by your lab will be shared not only with other sections, but also with future students, who will collect data at these same sites.
Abiotic Factors
Phosphate: Phosphate is an essential nutrient for both heterotrophs and autotrophs. Phosphate is often the limiting nutrient in freshwater systems. Natural levels of phosphate are 0.05 mg/mL. Poor water quality in many lakes and aquatic ecosystems is partly due to accumulated phosphate in the bottom sediments. Excessive phosphate can result in excessive algae growth (algae blooms) which is one cause of the fish kills the University Lake system periodically experiences. Algae blooms can release toxins, block sunlight, thus reducing lake autotrophs, and reduce dissolved oxygen. Phosphate occurs naturally in rocks and soil. Sources of excess harmful phosphate are fertilizers, animal feed, animal waste, and sewage.
Nitrogen Cycle Components: The movement of nitrogen in various chemical forms through the environment.
Ammonia (NH4+): Nitrogen-fixing bacteria convert nitrogen gas to ammonia, which can then be converted to the form used by plants. Ammonia results from metabolic waste and bacterial decay of organic material. Nitrogen fertilizers introduce ammonia into the environment.
Nitrite (NO2): A short-lived form of nitrogen intermediate between ammonia and nitrate. Chemotrophic bacteria convert ammonia to nitrite, and then other species of bacteria convert nitrite to nitrate.
Nitrate (NO3): The form of nitrogen that is usable by plants, where it can be converted to nitrogen-containing amino acids for use by heterotrophs.
LABORATORY ANALYSIS PROCEDURES ON COLLECTED WATER SAMPLES
Prepare a results table in your laboratory notebook for the analysis you will complete today, using the following template as a guide.
Table 6-1. Water quality data for test site _____________________on date _______________ .
Phosphate Test
Materials
Square mixing bottle
Two large viewing tubes with caps
Color comparator
PhosVer 3 phosphate reagent
Sample water
Methods
Wear gloves and safety goggles.
- Rinse a viewing tube with collected water. Discard rinse in untreated sample waste container.
- Fill the square mixing bottle to the 20 mL mark with collected water.
- Add contents of one PhosVer 3 phosphate reagent to the sample bottle.
- Swirl to mix. (All powder may not dissolve.)
- Incubate 8 minutes. A blue-violet color will develop if phosphate is present.
- Fill viewing tube to the top line with sample solution.
- Insert tube into top right opening of color comparator. Tube will not slide completely to rack bottom.
- Fill second viewing tube to top line with collected water.
- Insert tube into top left opening of color comparator.
- Hold up to light with the tube tops pointing toward the light source and view through the openings in the front of the comparator. Be careful not to spill from the tubes.
- Rotate the disc to obtain a color match. Determining the closest color match is essential to determining an accurate value.
- Divide the reading in the scale window by 50 to obtain the mg/L phosphate.
- Dispose of treated sample in phosphate test waste container. Dispose of untreated sample in untreated sample waste container.
- Rinse tubes and mixing bottle with tap water and return with rack for the next group.
Ammonia Test
Materials
Color comparator
Two test tubes
Ammonia salicylate reagent
Ammonia cyanurate reagent
Sample water
Dropper for adding sample to tubes
Methods
Wear gloves and safety goggles.
- Rinse one treated and one untreated viewing tube with approximately 5 mL water sample to be tested. Discard in untreated water waste.
- Fill one treated and one untreated viewing tubes to the 5 mL mark with water sample.
- Add contents of one foil packet of ammonia salicylate powder to treated tube (open with scissors).
- Swirl tube until powder dissolves.
- Incubate at room temperature for 3 minutes.
- Add contents of one foil packet of ammonia cyanurate powder to sample in treated tube.
- Incubate at room temperature for 15 minutes.
- Insert untreated sample tube in color wheel slot for untreated sample (left).
- Insert treated sample tube in color wheel slot for treated sample (right).
- Rotate the disc to obtain a color match. Determining the closest color match is essential to determining an accurate value.
- Read the results as mg/L ammonia nitrogen (1 ppm = 1 mg/L). The background due to the control sample is automatically subtracted.
- Discard untreated water in sink or untreated water beaker.
- Discard treated water in ammonia waste container.
- Rinse tubes with dH2O and discard in ammonia waste if colored.
- Return all materials to kit.
Nitrite Test
Materials
Two large viewing tubes with caps
Color comparator
Dropper for adding sample water to tube
NitriVer 3 nitrite reagent
Sample water
Methods
Wear gloves and safety goggles.
- Rinse a viewing tube with collected water. Discard rinse in untreated sample waste container.
- Fill the tube to 5 mL mark with collected water sample (bottom of frosted section).
- Add the contents of one NitriVer 3 nitrite reagent to sample tube.
- Stopper the tube and shake for 1 minute. (All powder may not dissolve.)
- Incubate 10 minutes. A pink color will develop if nitrate is present.
- Insert tube into top right opening of color comparator.
- Place a viewing tube with untreated sample water in top left slot of the comparator.
- Rotate the disc to obtain a color match to the tubes. Determining the closest color match is essential to determining an accurate value.
- Read the ppm (mg/L) nitrite nitrogen through the scale window.
- Record the results as mg/L nitrite nitrogen (1 ppm = 1 mg/L). The background due to the control sample is automatically subtracted.
- Dispose of treated sample in nitrite test waste container. Dispose of untreated sample in untreated sample waste.
- Rinse tubes with dH2O and place in rack for the next group.
Nitrate Test
Nitrate = nitrate test result − nitrite test result
Materials
Small test tube
Two large viewing tubes with caps
Color comparator
NitraVer 6 reagent
NitriVer 3 reagent
Sample water
Methods
Wear gloves and safety goggles.
- Rinse a small test tube with sample water. Discard rinse in untreated sample waste container.
- Add 5 mL of sample water to rinsed small test tube.
- Open one NitraVer 6 reagent and add contents to sample.
- Gently shake for 3 minutes.
- Incubate for approximately 30 seconds (if you don’t have a watch or a timing app on your phone, count to 30).
- Pour sample into a viewing tube for treated sample carefully. Do not allow settled cadmium particles to transfer to the new tube. Rinse small tube with tap water and dispose of rinse in nitrate test waste container.
- Add contents of one NitriVer 3 reagent to sample in viewing tube with sample for treatment.
- Gently shake for 30 seconds.
- Incubate 10 minutes. A red color will develop if nitrate is present.
- Insert tube into top right opening of color comparator.
- Fill second viewing tube with collected water to the mark. (This does not need to be exact.)
- Place in top left slot of the comparator.
- Hold up to light and view through the openings in the front of the comparator. Rotate the disc to obtain a color match. Read and record the mg/L nitrate nitrogen through the scale window. Determining the closest color match is essential to determining an accurate value.
- Dispose of treated sample in nitrate test waste container. Dispose of untreated sample in appropriate container. Rinse tube two times with dH2O and return to kit for the next class.
Post-Lab Quiz
Proceed to the Post-Lab Quiz