Chapter 2. DIVERSITY I—BACTERIA, ARCHAEA, PROTISTA
Learning Objectives
General Purpose
Conceptual
- Be able to compare and contrast the diversity within the domains Bacteria, Archaea, and Eukarya.
- Be able to describe the major evolutionary trends in each kingdom.
- Be able to describe the important structures and lifestyle differences in each major group.
Procedural
- Be able to describe and recognize bacterial shapes.
- Be able to identify and properly categorize organisms as members of Bacteria, Archaea, or Protista.
- Be able to use the microscope to observe and identify microscopic organisms and structures.
- Be able to convert the microscope from bright field to dark field and back.
Background Information
The organisms that comprise the domains Bacteria and Archaea were once classified together in the kingdom Monera. Organisms in both domains have prokaryotic cell types. Comparisons of the molecular components of these organisms has revealed that the Archaea are more evolutionarily related to organisms within the domain Eukarya than they are organisms in the domain Bacteria (Figure 9-4).
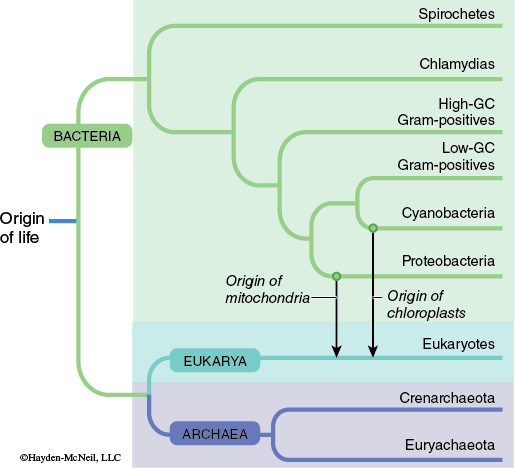
Even though members of the Bacteria and Archaea are classified in different domains there are many similarities between them in terms of basic cell structure and morphology. The members of these domains are largely unicellular and feature prokaryotic cell structure. These cells lack most membrane-bound structures such as a nucleus, mitochondria, and chloroplasts. Since prokaryotic organisms lack a true nucleus, they do not undergo cell division using mitosis or meiosis. Cell division in prokaryotes occurs through a mechanism known as binary fission.
Although the prokaryotes are often thought of as primitive or simple organisms, the Bacteria and Archaea display a tremendous diversity in metabolic and nutritional pathways.
Owing to the size (1–5 μm—in comparison, a dollar bill is just over 100 μm thick) of the Bacteria and Archaea, you will need to use a compound microscope to observe the specimens. The compound microscope is probably the single most commonly used instrument in biological laboratories. This type of microscope uses two separate lenses, an objective lens and an eyepiece (or ocular) lens to produce high magnification light microscopy images.
Exercise 1. Orienting Through the Microscope
Materials
Compound microscope
Microscope slide with the letter “e”
PROCEDURE
- Place the slide containing the letter “e” on the stage of the microscope (be sure the low power objective is in place) and bring it into focus using the coarse focus knob.
Answer the following questions in your laboratory notebook:
Is the image you observe right side up or upside down relative to the letter on the slide?
While you are observing the image, use the stage adjustment knob and move the slide to the left.
Which way does the image move?
Now use the stage adjustment knob and move the slide away from you. Which way does the image appear to move?
Change from the low power to the medium power objective. Is the size of the letter image larger or smaller?
Is the area observed in the field of view larger or smaller?
Repeat this process with the high power objective (40×).
Is the image larger or smaller?
Is the field of view larger or smaller?
If you were searching a new slide for a single microscopic organism, would it be faster to scan the entire area using the low power or high power?
Diversity of Bacteria
Background Information
The members of the domain Bacteria are classified into several phyla. A partial cladogram of some of these phyla is shown below (Figure 9-5).

Bacterial cells exhibit a variety of shapes. Individual bacterial cells can be one of three different forms: cocci (spherical), bacilli (rods), and spirilli (spirals or helical) (Figure 9-6).
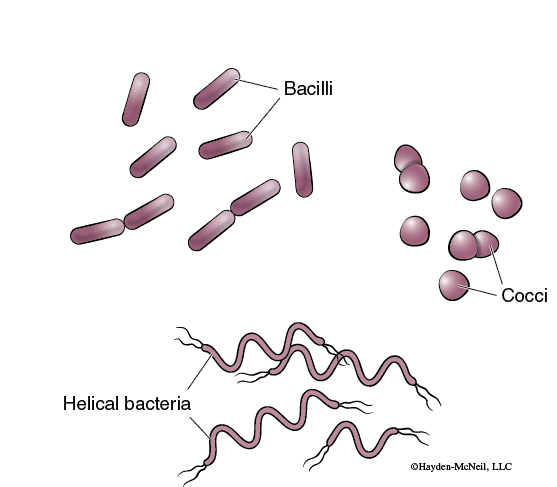
The shape of the cell is characteristic of the particular species and therefore is often useful when identifying unknown bacteria. A technique used by biologists to prepare bacteria for light microscopy is simple staining. This procedure allows the observation of size, shape, and arrangement of cells. A thin film or smear of the culture is placed on a microscope slide, fixed with heat to make the cells stick to the slide, and stained with a single stain.
Many bacteria are motile. Their movement is usually the result of the motion of a single bacterial flagellum. Bacterial movement can be very difficult to see except when using dark field microscopy.
Exercise 2. Diversity of Bacteria
Materials
Compound microscope
Microscope slide with bacteria of different shapes
Microscope slides with examples of the different Bacterial groups
Gram Positive
Chlamydias
Proteobacteria
Cyanobacteria
Spirochetes
Clean microscope slide
Hay infusion for living bacteria slide
PROCEDURE
Bacterial Shapes
- Obtain a prepared slide containing bacteria of different shapes.
- Examine the slide using the microscope. Focus initially using the low power objective (4×), and then switch to medium and high power objectives.
- In your laboratory notebook make a labeled sketch an example of each type of bacterium observed at high power.
Bacterial Diversity
- View a specimen from each of the different bacterial groups.
- Make a table in your laboratory notebook like the one that follows.
Table 9-1. Diversity of Bacteria.
- In the table write a brief description for each sample. Be sure to include the name of each sample.
Bacterial Movement
- Use a microscope set up for dark field. This set uses an inserted field stop (circular disk with the opaque center) in the filter holder of the microscope.
- Add a drop of infusion solution to a clean microscope slide and carefully place a cover glass on the drop.
- Focus initially using the low power objective (4×). Then switch to medium power and finally to high power.
- Look for swimming bacilli or spirilli. Choose the magnification that is easiest to observe the swimming.
- Observe the path of their swimming.
Answer the following questions in your laboratory notebook:
What type of path do they take?
What does this tell you about the movement of their flagellum?
Diversity of Protista
Background Information
Protists are eukaryotic, unicellular, filamentous, and colonial organisms. The size of protists varies greatly, with the smallest being about 1 μm and large colonies over 100 feet long. In general, the unicellular forms have a size in the range of 10–100 μm. They can be divided into three groups, based on their mode of nutrition. The heterotrophic protists ingest their food primarily by phagocytosis (the uptake of large particles or whole organisms by the pinching inward of the plasma membrane). The photosynthetic protists include all algae, except the green algae (now included with the plants). The third category includes the fungus-like slime molds that obtain their nutrition by absorbing nutrients from decomposing organic matter. The slime molds will not be addressed further.
Heterotrophic Protists
The heterotrophic protists are often called protozoa. There are three categories of protozoa based on their mode of locomotion. One group uses cellular extensions called pseudopodia, which includes organisms in the phyla Rhizopoda, Foraminifera, and Actinopoda. Another group uses flagella and includes members of the phylum Zoomastigophora. A third group uses cilia, which includes members of the phylum Ciliophora.
Protozoa That Use Pseudopodia
Phylum Rhizopoda
In this phylum, organisms have no fixed body shape, and they are naked. They do not have a shell or hard external coating. They may be found in both freshwater and marine habitats.
Phylum Foraminifera
Foraminiferans, also referred to as forams, are an abundant ocean protist that can be so prevalent that the bottom sediment may be composed primarily of their shells, known as tests (Figure 9-7).
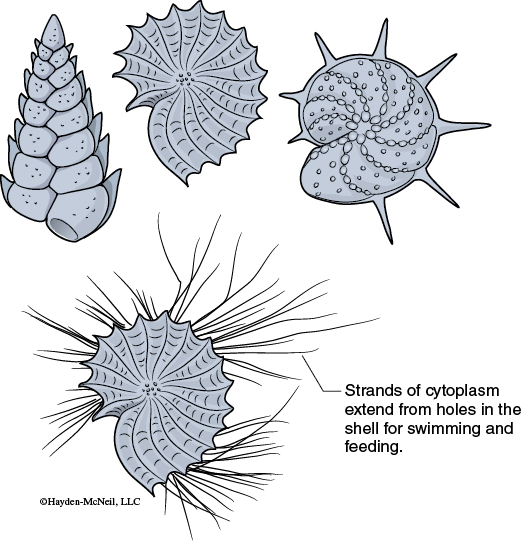
Most Foraminifera build their tests of crystalline calcite (calcium carbonate—CaCO3). Some tests are built of sediment material cemented together.
Phylum Actinopoda
Marine and freshwater organisms in the phylum Actinopoda have pseudopodia that are supported by a bundle of microtubules forming very slender axopodia. The axopodia extend from the central spherical cell body outward all directions through pores in the skeleton, which is formed from secreted silica.
Protozoa That Move Using Flagella
Phylum Zoomastigophora
Phylum Zoomastigophora consists of heterotrophic, flagellated protists. They may be free-living or they may live in parasitic symbiotic relationships with other organisms. One genus in this phylum, Trypanosoma sp. contains parasites that alternate between vertebrate and invertebrate hosts. One member of this genus T. gambiense, causes African sleeping sickness in humans.
Protozoa That Move Using Cilia
Phylum Ciliophora
The heterotrophic protozoa, Paramecium sp. is a common ciliate (Figure 9-8).
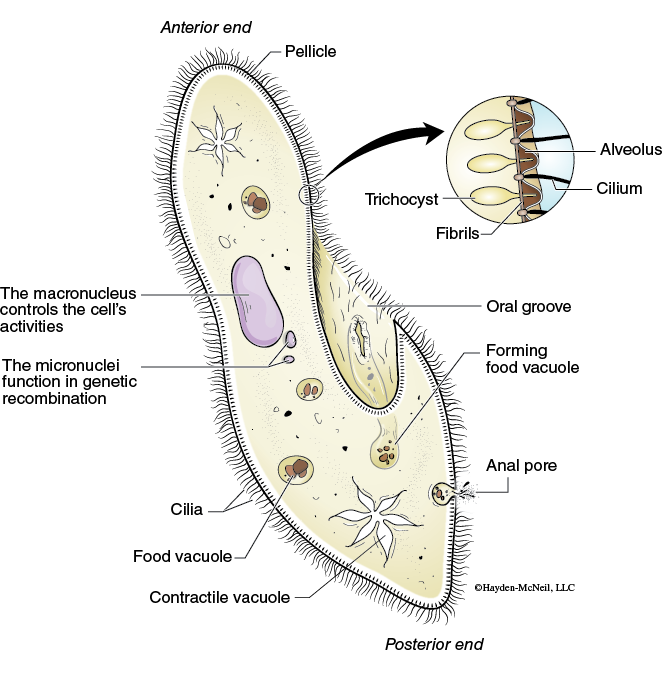
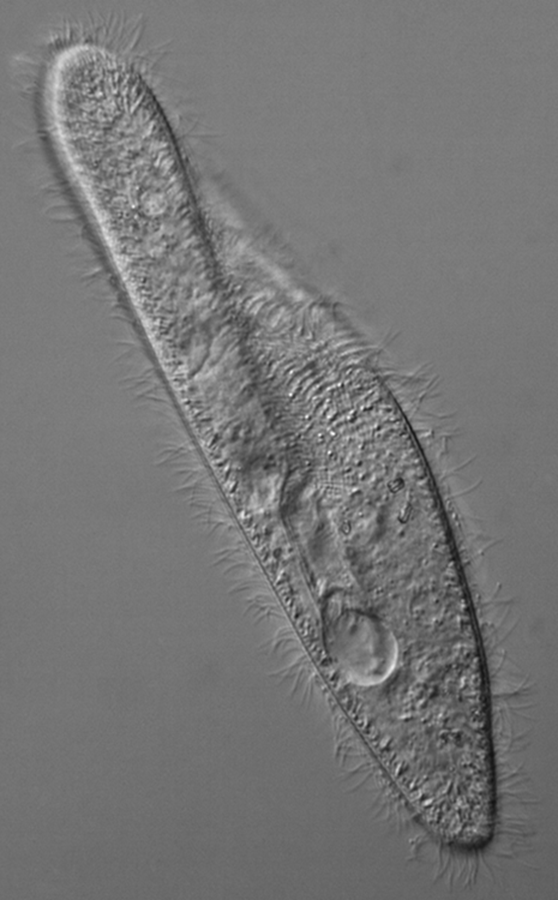
Members of the phylum ciliophora can be found in the most aquatic systems and are often observed in samples taken from the University Lake ecosystem. Their movement is distinguished from flagellates by its smooth motion in all directions, including its ability to reverse (back up) when encountering an obstacle.
Autotrophic Protists
The autotrophic protists are photosynthetic. This group is referred to by several non-taxononic names such as seaweed, algae, or phytoplankton. The various phyla are often distinguished by body form, presence or absence of flagella, and type of photosynthetic pigments.
Division Dinoflagellata
Dinoflagellates come in a variety of cell shapes and each organism has a pair of flagella (Figure 9-9).
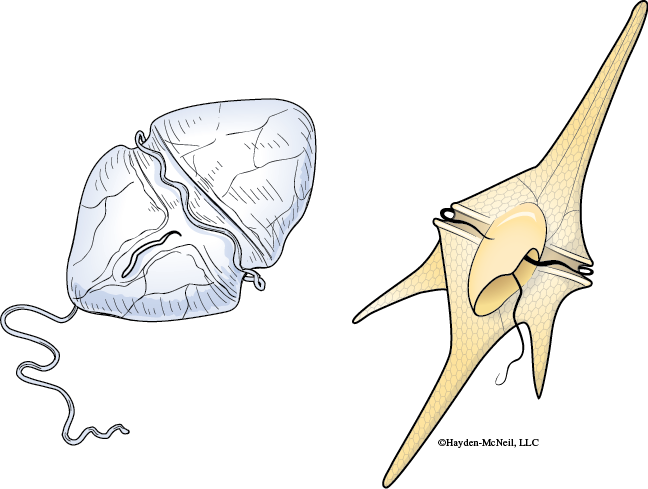
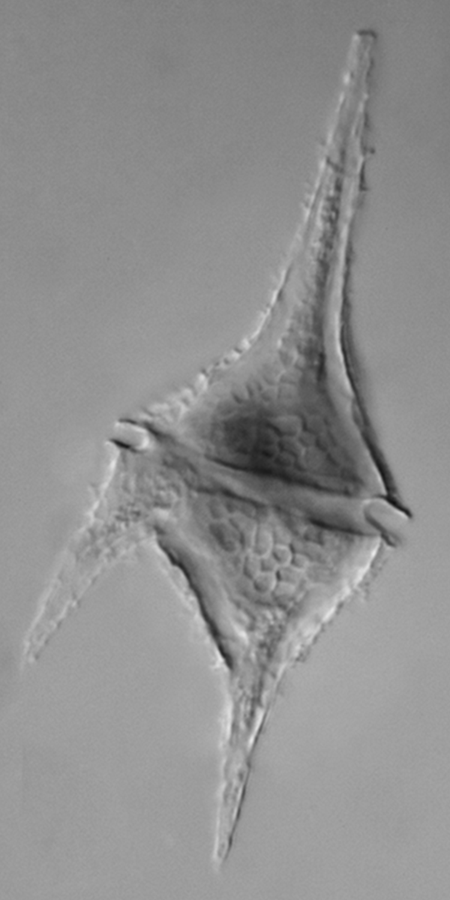
Dinoflagellates are often responsible for two unusual marine phenomena. First, much of the bioluminescence observed at night in the ocean is caused by dinoflagellates. Second, red tides are caused by large blooms of dinoflagellates (Figure 9-10).

Red tides are often associated with fish kills owing to products produced by the dinoflagellates themselves and or the reduction of oxygen which can result from the rapid decomposition of the die off of the algal bloom. These organisms play an important role in primary productivity (photosynthesis), which ultimately provides food for all marine organisms. They are also one of the chief producers of planetary oxygen.
Division Bacillariophyta
Members of the division bacillariophyta are often referred to as diatoms. Diatoms are responsible for much of the primary productivity in cold marine waters. Diatom cells are either elongated, bilaterally symmetrical pennate forms or radially symmetrical centric forms. The cell wall consists of two halves, one fitting inside the other, in the manner of the lid and bottom of a Petri dish.
Division Phaeophyta
Phaeophyta are commonly called brown algae. This is because some species (though not all) can appear brown because of the presence of the brown pigment fucoxanthin in addition to chlorophyll a. These algae are more closely related to diatoms than to green algae. Despite being in the Kingdom Protista, some of the largest algae, the kelps, are brown algae. The brown algae exhibit characteristic gas bladders. In addition, they can have many characteristics analogous to plants (multicellular organisms), including: root-like holdfasts, stem-like stipes, and large leaf-like blades. Aside from these apparent similarities to plants, the molecular biology evidence indicates the closest relatives of the brown algae are unicellular protists.
Phylum Rhodophyta
Red algae contain chlorophyll a and the accessory pigments phycocyanin and phycoerythrin that often mask the chlorophyll, making the algae appear red (Figure 9-11).

These accessory pigments absorb green and blue wavelengths of light that penetrate deep into ocean waters. Like the brown algae, the red algae also have commercial value. Agar and carrageen are extracted from the cell walls. Agar is used extensively for the culturing of bacteria. Carrageen is used to give the texture of thickness and richness to foods such as dairy, drinks and soups.
Exercise 1. Characteristics of Phylum Rhizopoda (Protozoa That Use Pseudopodia)
Materials
Microscope
Depression slide
Culture containing members of Phylum Rhizopoda
PROCEDURE
- Use the depression slide to make a wet mount containing one amoeba.
- Observe the organism first on the lowest power of the compound microscope and then on intermediate power.
- Record any observations in your laboratory notebook and make note of how the organism moves.
- In your laboratory notebook make a sketch of one or more views of the amoeba on the slide.
Exercise 2. Characteristics of Phylum Foraminifera (Protozoa That Use Pseudopodia)
Materials
Microscope
Prepared slide of a member of Phylum Foraminifera
PROCEDURE
- Obtain a prepared slide of representative forams.
- Observe the organisms first on the lowest power of the compound microscope and then at intermediate power.
- Record any observations in your laboratory notebook.
- In your laboratory notebook make a sketch of one or more of the forams that you observed.
Note that the chambers can be a single row, multiple rows, or arranged in a spiral. Biologists determine the Foraminifera species based on the arrangement and appearance of the tests.
Exercise 3. Characteristics of Phylum Actinopoda (Protozoa That Use Pseudopodia)
Materials
Microscope at a demonstration station with a prepared slide of Radiozoa (a member of Actinopoda)
PROCEDURE
- Observe the organisms first on the lowest power of the compound microscope and then at intermediate power.
- Observe the size and shape of the skeletons and record any observations in your laboratory notebook.
- Compare the observations of Actinopoda with the observations of Foraminifera. Note any similarities or differences.
- In your laboratory notebook make a sketch of the Radiozoa that you observed.
Exercise 4. Characteristics of Phylum Zoomastigophora (Protozoa That Use Flagella)
Materials
Microscope
Prepared slide of a member of Trypanosoma sp. (a member of Zoomastigophora)
PROCEDURE
- Observe the organism first on the lowest power of the compound microscope and then on intermediate and high powers. Note the organism is in a blood sample so you will have to locate the organism among the blood cells from the parasite’s host.
- Observe the organism and record any observations in your laboratory notebook.
- In your laboratory notebook, make a sketch of Trypanosoma sp. Make sure you can distinguish between erythrocytes (red blood cells), leukocytes (white blood cells), and Trypanosoma.
Exercise 5. Characteristics of Phylum Ciliophora (Protozoa That Use Cilia)
Materials
Microscope
Depression slide
Protoslo (methyl-cellulose solution)
Culture containing Paramecium sp. a member of Phylum Ciliophora
PROCEDURE
- Place a drop of water from the bottom of the Paramecium sp. culture on a clean depression slide. Add a small drop of Protoslo (methyl-cellulose solution), and then add the coverslip.
- Observe the organism using the compound microscope using low and then intermediate magnification.
- Record any observations in your laboratory notebook and make note of how the organism moves. Answer the following questions with your observations in your laboratory notebook: Does the movement appear to be directional or is it random? Does the organism reverse direction only when it encounters an object, or does it appear to reverse direction even with no obstruction?
- Locate a large, slowly moving organism trapped in the Protoslo, switch to high power magnification, and identify the following organelles and features:
Oral groove
Food vacuole
Macronucleus
Contractile vacuole
In your laboratory notebook make a sketch of the Paramecium sp.
- Observe the feeding behavior of the Paramecium sp. Add a drop of yeast that has been stained with Congo red to the edge of the coverslip and watch as it diffuses around the Paramecium sp. Make note of these observations in your laboratory notebook.
Exercise 6. Comparison of the Characteristics of Heterotrophic Protists
Now that you have observed representatives of the major heterotrophic protists make a table in your laboratory notebook using Table 9-2 as an example and fill in the information for each of the organisms.
Table 9-2. Characteristics of heterotrophic protists.
Exercise 7. Characteristics of Phylum Dinoflagellata (Autotrophic Protists (Algae))
Materials
Microscope
Depending on availability you will use a prepared slide or make a wet mount from a culture
Prepared slide of a member of Phylum Dinoflagellata
Depression slide
Culture containing a member of Phylum Dinoflagellata
PROCEDURE
- Obtain a prepared slide or make a wet mount of dinoflagellates.
- Using low power attempt to locate cells. You may have to switch to medium power to see them clearly.
- Once the organism has been located switch to high power.
- Identify the perpendicular grooves and cellulose plates making up the cell wall. Record any observations in your laboratory notebook and if you are using a living specimen make note of how the organism moves. Answer the following questions with your observations in your laboratory notebook: Are the plates in your species elongated into spines? Flagella may be visible in living specimens (Figure 9-9).
- In your laboratory notebook make a sketch of the dinoflagellates species you observe.
Exercise 8. Characteristics of Phylum Bacillariophyta (Autotrophic Protists (Algae))
Materials
Microscope
Depression slide
Culture containing a member of Phylum Bacillariophyta
PROCEDURE
- Prepare a wet mount from the culture containing a member of Phylum Bacillariophyta.
- Using the compound microscope observe the organisms on low, intermediate, and high power.
- Record any observations in your laboratory notebook and describe the form of the diatoms in your sample. Are they centric, pennate, or both?
- In your laboratory notebook make a sketch of one or more views of the diatoms on the slide.
- Examine a prepared fixed slide of diatoms. Record any observations in your laboratory notebook and make a sketch of one or more views of the diatoms on the prepared slide.
Exercise 9. Characteristics of Phylum Phaeophyta (Autotrophic Protists (Algae))
Materials
Demonstration station with several members of Phylum Phaeophyta
PROCEDURE
- Observe all of the examples of brown algae that are on demonstration.
- Record any observations in your laboratory notebook and describe the various forms of the species of Phylum Phaeophyta.
- In your laboratory notebook make a table using Table 9-3 as an example and fill in the information for each of the organisms.
Table 9-3. Observations of several species of Phylum Phaeophyta.
Exercise 10. Characteristics of Phylum Rhodophyta (Autotrophic Protists (Algae))
Materials
Demonstration station with several members of Phylum Rhodophyta
PROCEDURE
- Observe all of the examples of red algae that are on demonstration.
- Record any observations in your laboratory notebook and describe the various forms of the species of Phylum Rhodophyta.
- In your laboratory notebook make a table using Table 9-4 as an example and fill in the information for each of the organisms.
Table 9-4. Observations of several species of Phylum Rhodophyta.
Post-Lab Quiz
Proceed to the Post-Lab Quiz