Chapter 4.
Overview
Generality
It can be plausibly argued that the most important and useful model in all of science is the particle model of matter. Here we formulate the particle model in a simple uncomplicated way, yet will see that it is extremely powerful in helping us to make sense of and understand a vast array of phenomena.
A famous Nobel laureate in physics, Richard P. Feynman, in the first chapter of an introductory physics book he wrote for Cal Tech students back in the late 50’s, claimed that the particle model of matter is the most important or powerful model in science. Here is what he said:
“If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement would contain the most information in the fewest words? I believe it is the atomic hypothesis (or the atomic fact, or whatever you wish to call it) that all things are made of atoms—little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another [emphasis added]. In that one sentence, you will see, there is an enormous amount of information about the world, if just a little imagination and thinking are applied.”
Focus
Our focus with this introductory particle model of matter is on the highlighted portion of the above quote:
“…little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another.”
Our job is to supply the structure and background that you, the learner need, to be able to apply the “little imagination and thinking” required be able to use, that is, to apply these simple ideas of the model to make sense of and answer questions about various kinds of phenomena. Specifically, we need ways to analytically think about and reason with the notion of particles attracting when close enough to each other, but repelling when squeezed together. That is what this model is about.
Model Summary

Representational Tools
Algebraic Representations
The magnitude of the attractive or repelling force between two particles is:
|F| = |d(PE)/dr|.
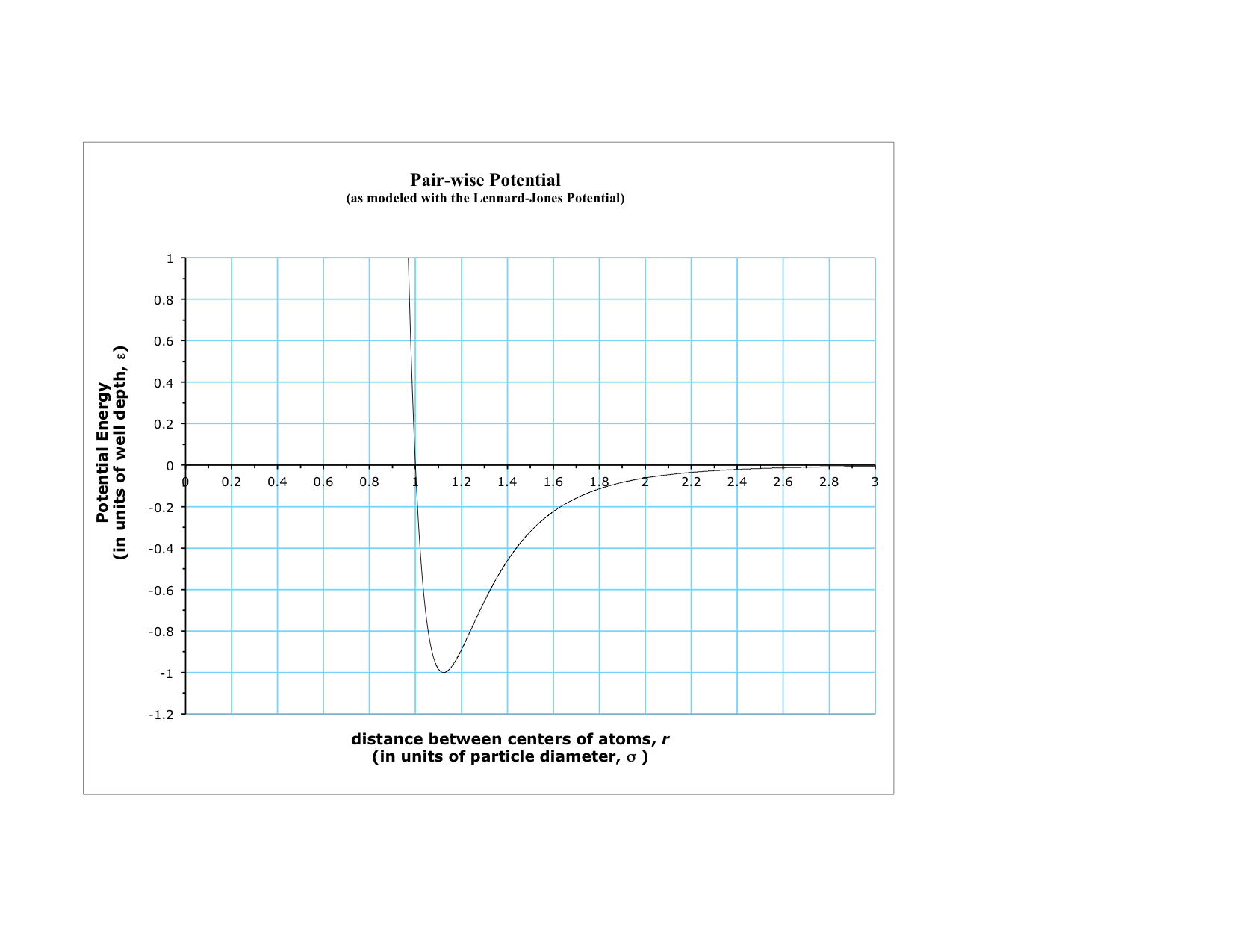
Graphical Representation of the particle-particle pair-wise Potential Energy
Elaboration
Expanded Definitions of the Model Constructs
Particle
This label applies to microscopic constituents of matter, typically an atom or molecule, but it could also refer, for example, to the constituents of the nucleus, if that were the focus of interest.
Attractive and Repulsive Forces
Atomic sized particles exert forces on each other in the same way that large-scale objects do. These forces can be attractive or repulsive; for electrically neutral particles, whether the force is attractive or repulsive depends on their separation.
Interaction between two particles
The basis for making sense out of how particles interact is to focus on the interaction of only two particles at a time. There are several properties that keep reoccurring in our description of this interaction.
Center-to-center separation
We consistently refer to the distance between particles as being the center-to-center separation, rather than the distance between their surfaces. Usually we will use the symbol r to indicate this separation distance.
Equilibrium position or equilibrium separation
When we are focusing on just two particles, we will find that there is often a “special” separation, often referred to as the equilibrium separation. The reason this separation is special is that at this separation the interparticle force is zero. What does this say about the slope of the PE curve at this point? It has similarities to the spring-mass system in this respect. A mass hanging on a spring hangs at a particular “separation” from the point at which the spring is supported. This is a favored position. If the mass finds itself closer to the point of support, the “spring force” pushes it away, back toward the equilibrium position. Conversely, if it finds itself too far form the support, the spring force pulls it back toward the equilibrium position. The exact same thing happens with two atomic sized particles. We label the equilibrium separation for two particles with the symbol ro.
Pair-wise Potential Energy
We will consistently refer to the potential energy between two atomic size particles as the pair-wise potential energy, PEpair-wise. This potential energy has a fairly generic shape to it that you need to become familiar with. In general terms, it becomes very repulsive if the two particles begin to get too close to each other. The potential has a minimum and becomes “horizontal”–slope is zero–at the two particle’s equilibrium separation (i.e. the equilibrium chemical bond length for these two particles). As the particles begin to separate, the potential at first “looks like” a spring-mass potential, but then begins to flatten out and becomes perfectly flat (horizontal, so zero force acting between the two particles here) once the separation is a few times that of the equilibrium separation, ro. The parameter that describes how “deep” the potential is, that is, the difference in energy between where the potential is flat at large separations and at its lowest value where the equilibrium separation occurs, is often called the “well depth” and designated with the lowercase Greek letter ε. The well depth, ε, is the magnitude of this energy difference, so is always a positive quantity.
Single Particle Potential Energy
In a solid or liquid, each particle has multiple pair-wise interactions, because it has lots of neighbors to interact with. It will sometimes be useful to focus on just one particle at a time, and to “add up” all the interactions it has with its neighbors to obtain a potential energy function that describes the forces acting on just this one particle from all of its neighbors. We call this the single-particle potential energy to make it clear that it is not PEpair-wise.
Discussion of the Model Relationships
Relationship 1
All “normal” matter is comprised of tiny particles (atoms and molecules) that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another. (paraphrased, Feynman)
This is a slightly paraphrased quote from Nobel Laureate Richard Feynman in which he stated that if all scientific information were to be lost, these would be the most valuable ideas to pass on to future generations. The point is that electrically neutral particles exert a force on each other. The force will be repulsive if the particles are squeezed into each other. The force will be attractive when they are close to each other, but not “squeezed into each other.” And, not explicitly stated, but implied, the particles will not exert a force on each other with they are not close to each other.
Relationship 2
The attractive or repelling force is related to the shape of the potential energy curve characterizing the particle interaction. In one-dimension, the magnitude of the force is equal to the derivative of the potential energy with respect to the particle separation. The force is always in the direction that lowers the potential energy.
|F| = |d(PE)/dr|.
Relationship 3
The pair-wise potential energy for particles has the general shape shown in the graph. Important features of the force acting between two particles are revealed by applying the relationship listed in (2) above to the graph.
- strongly repulsive when the two particles are separated by a distance less than that corresponding to the minimum of the potential.
- attractive when the particle separation is greater than that corresponding to the minimum of the potential.
- attractive force is “short range,” quickly approaching zero as the particle separation increases.
The particular curve shown is known as the Lennard-Jones potential, and is an excellent approximation for noble gas atoms. All pair-wise potential energies (except for like-charged ions) have this general shape with these features (this includes all types of chemical bonds).
It is very important the you can reliably visualize the pair-wise potential energy curve and can explain the three points in this relationship in terms of the derivative/slope of the curve.
Relationship 4
When there are many particles, the phase (s, l, g) of those particles depends on their total energy. At sufficiently high total energy, the particles are unbound and in the gas phase. At sufficiently low energy the particles are in the liquid or solid state and are bound. The average particle-particle separation in the bound state is approximately equal to the separation corresponding to the minimum of the pair-wise PE. In the unbound state it is much greater than the separation corresponding to the minimum PE.
One common mistake that many students make is to attempt to ascribe macroscopic properties (like solid, liquid, gas) to the interaction of only a small number of particles using PEpair-wise. The macrostate of matter, whether it is in a solid, liquid or gas phase, for example, is due to the simultaneous interactions of something like 1025 pair-wise interactions assuming we have a mole of the substance. These ideas are not easy, so be patient. Initially, try to imagine a solid at very low temperatures. Each particle “wants” to be at the right distance with respect to all of its neighbors. If there is a way for the system to “get rid” of its energy (by giving it to some colder system, for example), it will continue to settle down and reduce its thermal energy. Eventually, all the random motion comes to a stop (if we can keep cooling the sample) and the particles find their “magic” places, each near the “bottom” of the PEpair-wise with each of its neighbors.
Now, imagine that we start adding energy to the sample. All the particles begin acting like little spring-masses, oscillating back and forth around their equilibrium positions. Eventually they move sufficiently far, so that some “jump” out of where they are “supposed to be.” Particles at or near the surface might even leave the sample if their vibrations get vigorous enough. Picturing what happens when a substance melts, i.e., turns from a solid to a liquid, is difficult, even for the experts. Don’t worry about picturing that transition. But you can imagine continuing to add energy until all the particles, even 1023 particles, have sufficient energy to separate far apart from each other, causing them to be in the gas phase. Recall what value all ~1025 pair-wise potentials will have, if all particles are separated by many particle diameters. So, what is the bottom line here at this point in our making sense of all this? Without getting into a lot of detail, it should make sense to you that at some sufficiently low temperature, everything will be a solid and at some sufficiently high temperature, everything should be a gas. That is plenty for right now.
Relationship 5
The interactions of one particle in a liquid or solid with all of its neighbors add together to form one three-dimensional potential energy for a particular particle with a minimum that defines the equilibrium position of that particle (where the net force due to all of the pair-wise interactions is zero). We refer to this potential as the single-particle potential energy to emphasize its distinction from the pair-wise potential energy.
We don’t need to go any further than to simply visualize that such a single-particle potential exists for each particle in the condensed state of matter (liquid or solid).
Relationship 6
Each particle in a solid or liquid oscillates in three dimensions about its equilibrium position as determined by its single-particle potential.
OK, so here is where we are attempting to make our mental picture a little clearer regarding what is happening to a single particle (which could be an atom or a tightly bound-together molecule) when it finds itself somewhere in the middle of similar particles in a solid or liquid. It really is acting like it is attached to a bunch of springs with all of its neighbors (and nearby neighbors). But here is the “really neat” thing. No matter how complicated the actual chemical bonds are, and no matter how many there are, or in what directions they point, they all add up to exactly what would happen if you had only three (that’s right, only three) little springs of exactly the right strength, one going out in each of the three x, y, and z directions of the three-dimensional space we seem to occupy in this universe (at least on our scale and on the scale of atoms and molecules). So the picture you want to get into your head is something like that shown below, remembering that the spring constant of the springs can be different in the three directions.
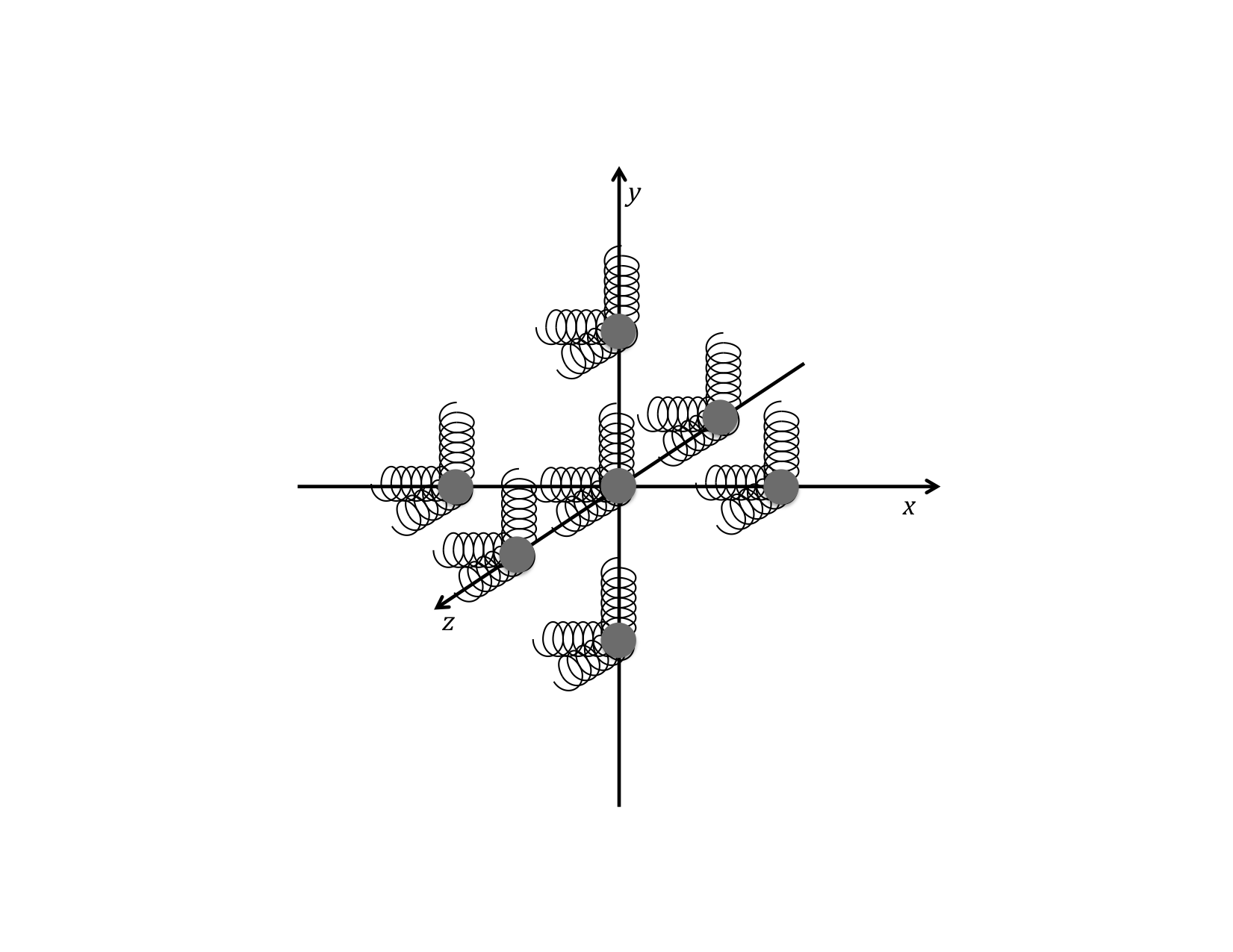
We will come back to this picture shortly when we make more sense of thermal energy.
But, what about the bond energy? Well, it really does depend on the real bonds, the real chemical bonds. However, we can develop reasonable estimates in terms of the well depths of the pair-wise interactions for the bond energy that work for practically all pure substances. Carrying out the analysis to make sense of bond energy and to make sense of thermal energy is what the next two models are about.