Chapter 1. Experiment 1
Physical and Chemical Properties
Purpose of the Experiment
Determine the densities of the liquids (physical property) and to determine the solubility of a solid in several liquids (chemical property) and the miscibility of the liquids with each other (chemical property).
Background Required
This experiment will use basic laboratory techniques in measuring mass and volume. The concepts of physical and chemical properties, solutions and mixtures, solvents and solutes are used in this experiment.
Background Information
In our everyday life, we are constantly making solutions and mixtures. When we add sugar to our coffee or tea, the solid dissolves in the liquid to form one solution. The ability of a solid or gas to dissolve in a liquid is its solubility. So the sugar is soluble in the water/coffee solution. The solution that does the dissolving is the solvent. The substance being dissolved is the solute. Because this property depends on its chemical nature or reaction, it is a chemical property.
If we add cream to our coffee, the result is a solution that contains the properties of both liquids. It is no longer just coffee or cream, but one solution that has the flavors of each. When two liquids mix completely with each other, they are miscible. If two liquids do not mix completely, they will form layers. Then the liquids are described as immiscible. An example of immiscible mixture is the combination of olive oil and vinegar in an oil vinaigrette for salad. No matter how much you shake the mixture, eventually two layers will form because the olive oil and vinegar are immiscible with each other.
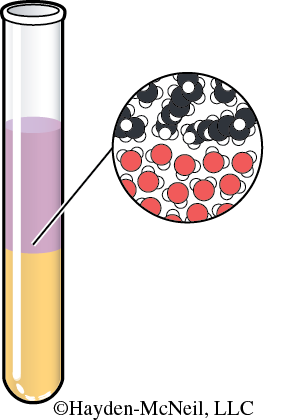
After stirring the oil and vinegar mixture, the oil layer always ends up on top. Why is that? A substance’s density is its mass to volume ratio (mass/volume). The layer with the lesser density will float or rise to the top of the denser layer.
This is also seen with solids and liquids. Ice is less dense than water, so an ice cube will float in a glass of iced tea. On the other hand, a spoon will sink to the bottom of a pan of dishwater. That is because the metal spoon is denser than water. (Density is an example of a physical property.)
Example
Problem
The mass of 3.8 mL of Solution A is 4.13 g. Determine density of Solution A.
Solution
You need the equation for density.
Density = mass/mL
Density = 4.13 g/3.82 mL
Density = 1.08115 g/mL (next step, round to 3 significant figures)
Density = 1.08 g/mL
In This Experiment
In this experiment you will determine the densities and miscibility of several liquids with each other, and the solubility of a solid with the liquids.
Review of Some Laboratory Techniques and Procedures
Since chemicals are never to be placed directly on the weighing pan of a balance, a technique of weighing by difference is used. This technique involves determining and recording the mass of an empty container, then determining and recording the mass of the container + chemical. The difference between the two masses is the mass of the chemical.
Most electronic balances also have a tare feature. This recalibrates the balance to read zero with the weight of the empty container currently on the balance. Then add the chemical to the container and reweigh. This feature works well if you can add the chemical immediately.
To dispense a desired volume, pour a small volume into a small beaker from the main reagent bottle. (Only obtain a volume slightly greater than the volume to be used.) Then you can either pour from the small beaker or use a disposable Beral pipet to obtain the amount needed from the beaker.
Never pour the extra solution back into the main reagent bottle. You can share with another group or pour into the waste container.
In volume measurements, it is very important to correctly and consistently read the bottom of the meniscus. To do this, you must be at eye level with the meniscus. You will also have to estimate the last digit of the volume measurement.
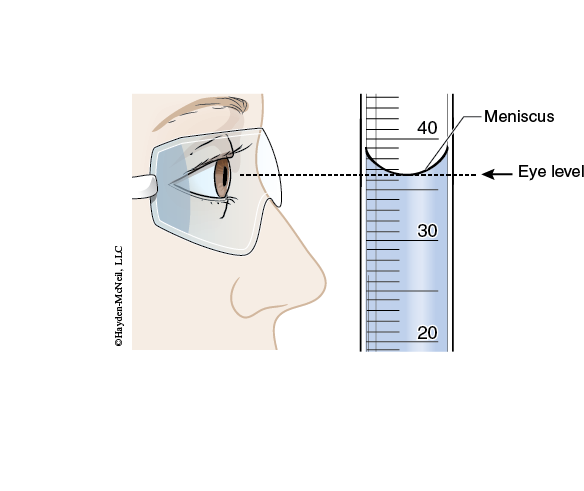
All data is always to be recorded directly in your laboratory notebook pages.
- All mass measurements will be recorded with 2 digits past the decimal.
- All volume measurements will be recorded with 2 digits past the decimal. Remember, estimate the last digit of the volume measurements.
Procedure
Physical and Chemical Properties
Always Wear Safety Goggles and Use Good Lab Practices
Chemical Alert:
Part I
Determining the Density of Three Solutions
1. Label five small test tubes as C6H14, CH2Cl2, H2O, Mixture 1, and Mixture 2.
2. Using the weighing by difference technique, weigh and record the mass of a clean, dry 10 mL graduated cylinder.
Pour between 4–6 mL of hexane into the graduated cylinder.
You are not trying to obtain a specific volume, just in the range of 4–6 mL.
Record the exact volume in the graduated cylinder.
Reweigh and record the mass of the cylinder 1 hexane.
3. Pour the hexane into the appropriately labeled test tube.
4. Repeat Step 2 to determine the mass and volume of the dichloromethane and water samples.
Pour each liquid into the appropriately labeled test tube.
5. Observe and record the physical characteristics of each liquid.
Calculate the density of each liquid.
Part II
Determining Solubility and Miscibility
6. Add 1–2 iodine crystals to each test tube and stir well with a glass stirring rod. Record your observations.
Did the iodine crystals float or sink? Did the crystals dissolve? Do the solutions change in appearance?
7. Using a new Beral pipet for each solution, transfer 40 drops of the densest liquid into the test tube labeled Mixture 1.
Transfer 40 drops of the next densest liquid, and then the least dense liquid to the same test tube.
Record your observations. You may want to draw a diagram.
How many layers formed? Did any of the layers shift positions after adding another solution?
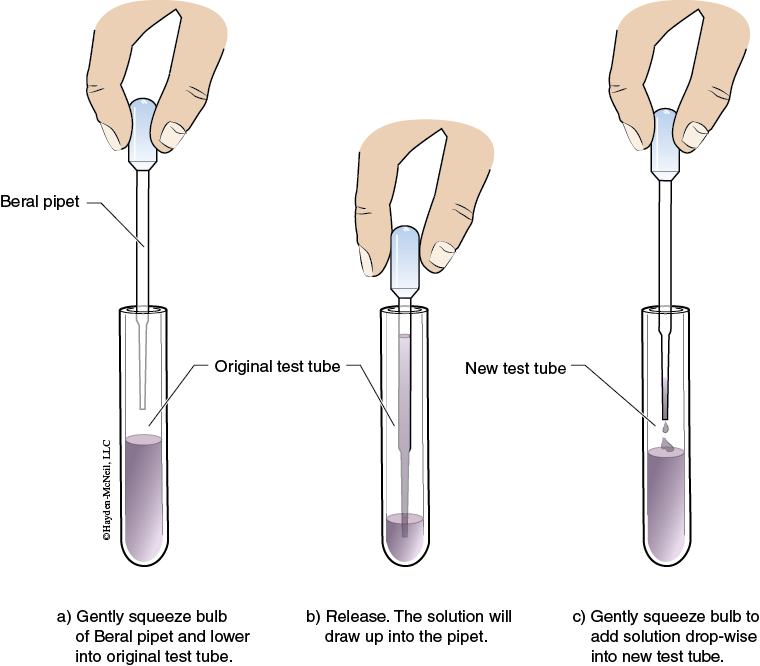
8. Transfer 40 drops of the CH2Cl2 solution using a Beral pipet into the test tube labeled Mixture 2. Next, add 40 drops of the C6H14 to the test tube.
Record your observations.
Finally, add 40 drops of water to the Mixture 2 test tube.
Record your observations. Are the observations the same or different from Mixture 1?
9. Pour all of the solutions into the waste container in the hood.
Wash all used glassware with detergent, rinse with water, and allow to dry.
Do not force towels into the test tubes or graduated cylinder to dry out the insides of the glassware.
10. ALWAYS WASH YOUR HANDS AFTER FINISHING AN EXPERIMENT. There is hand soap available at the sink at the back of the lab.
Determinations
From your observations, what can you infer about the solubility of I2 in each of the liquids?
From your observations, what can you infer about the miscibility of the three liquids with regard to each other?
Below is a sample portion of what should be recorded in the lab notebook pages found immediately behind the procedure. The lab notebook should have all of your data, observations, and any determinations or calculations.
Physical observations of liquids
Observations after adding I2:
Inferences (what can you deduce?):
Observations after transferring the liquids into Mixture 1:
Inferences (what can you deduce?):
Observations after transferring the liquids into Mixture 2:
Inferences (what can you deduce?):
Calculations
All work must be shown on your notebook pages.
If any solutions combined into one layer in the test tubes labeled Mixtures 1 and 2, you will need to calculate the average density of the resulting mixture. Since you added the same number of drops of each liquid, this will be a simple average.
Discussion
For this first experiment, the guidelines for the discussion section are given. However, as you gain more experience in writing scientific reports, these guidelines will not be given.
- Summarize the results of this experiment. What did you set out to do in the purpose of the experiment—what were the densities of the liquids, in which liquids did the solid I2 dissolve, and which liquids were miscible with each other?
- What is the quality of the results? Do the results seem reasonable? How did the determined density of water compare to the known density, 1.0 g/mL of water?
- What were the errors or possible errors in the experiment? List any errors or problems in the procedure. Also list some possible errors in the experiment. Example: Reading down on the graduated cylinder instead of reading up, so that the volume was recorded as 6.65 mL instead of 5.35 mL.
- How would the errors affect the results of the experiment? Example: Misreading the graduated cylinder volume as 6.65 instead of 5.35 will affect the density calculation. Since the mass will be divided by a larger volume, the density will be smaller than true density.
Study Questions
(These Study Questions will not be turned in or graded; however, they will help you study for the upcoming Quizzes on this experiment.)
1. Classify each of the following as either a physical or chemical property.
a. melting point
b. ability to dissolve metals
c. ability to react with oxygen
d. boiling point
e. color of liquid
f. ability to dissolve in water
2. What would happen if you mixed water, olive oil, and an ice cube in a beaker? (Read the introduction for background information.)
Draw a picture and label the layer(s).
3. Describe the mixtures as soluble, insoluble, miscible, or immiscible.
a. Al2O3 sinks to the bottom of a tall column filled with water.
b. Sand settles to the bottom of a pond.
c. Water and acetone mix completely.
d. Salt completely disappears when added to boiling water.
e. MgSO4 remains a solid when mixed with hexane (ℓ).
4. How would the value of density for water be affected if the mass of the water was recorded as 5.71 g instead of 5.17 g for a volume of 5.22 mL? (Will it be higher, lower, or no effect?) Explain your answer.
5. Rubbing alcohol is miscible with water and has a density of 0.785 g/mL. Olive oil is immiscible with alcohol and has a density of 0.92 g/mL.
What will happen when water is added to a beaker, then the olive oil, and then rubbing alcohol?
Draw a diagram and label the layer(s).
What will happen if the layers are stirred and then allowed to settle?
Draw a diagram and label the layer(s).
6. Use the data given below to draw a diagram and label the location of the compounds when they are mixed together in a beaker. The two liquids are immiscible and the solid is insoluble in both liquids.
7. An experimental method to estimate the density of a crystal is to compare it to a variety of known densities. Solution J (density = 1.9 g/mL), Solution K (density = 1.5 g/mL) and Solution L (density = 1.2 g/mL) are poured into a test tube. The liquids are immiscible with each other and solid M is insoluble in each. When a few crystals of M is added to the test tube, it sinks through solutions K and L, but floats on top of solution J. What is its approximate density?
Activity Completed!