Chapter 1. Experiment 13
Qualitative Inorganic Analysis: Group 3 Known
Purpose of the Experiment
To separate and identify representative cations from Qualitative Analysis Group 3.
Background Required
You should be familiar with techniques found in the Qualitative Inorganic Analysis Preview and with acid/base terms.
Background Information
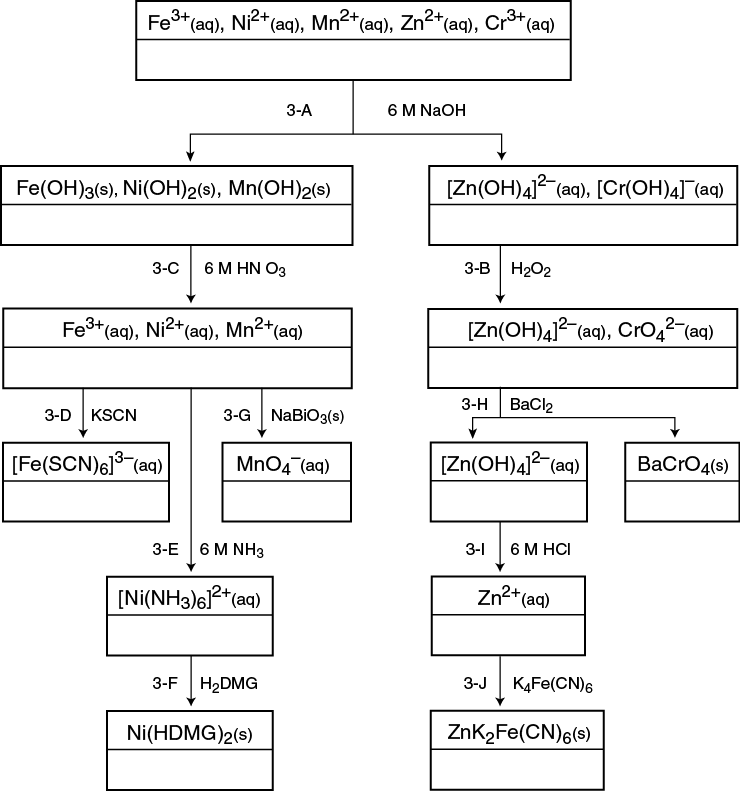
Group 3 cations precipitate as sulfide compounds from a basic solution (pH ≥ 9). To avoid using sulfide salts, this scheme has been modified to analyze the Group 3 cations separately from the other Groups. The seven cations of Group 3 are: Fe3+, Cr3+, Al3+, Co2+, Ni2+, Mn2+, and Zn2+. In this scheme, you will consider Fe3+, Cr3+, Ni2+, Mn2+, and Zn2+. These Group 3 cations are all first-row transition metals. In general, transition metal compounds and solutions are colored, and the solids and solutions in this scheme have distinctive colors. In the early steps with the known cation solution, you will be observing the colors of the combined cation compounds or ions. Therefore, steps must be taken to separate the species until the distinctive colors can be observed in the confirmation step. Carefully observe the colors in each step.
In this analysis, you must also pay careful attention to the acid/base conditions. Certain compounds, such as Cr(OH)3 and Zn(OH)2, are amphoteric. Amphoteric compounds are insoluble, but dissolve when the solution is made either strongly acidic or basic. So you must follow the instructions explicitly when adjusting the pH of the solutions.
Group 3 Procedure Background
3–A: Separation of [Zn(OH)4]2– and [Cr(OH)4]–
In this step, NaOH is added to precipitate Fe(OH)3, Ni(OH)2, Mn(OH)2, Zn(OH)2, and Cr(OH)3. However, the addition of excess NaOH will dissolve the amphoteric Cr(OH)3 and Zn(OH)2 to form soluble complex ions, [Zn(OH)4]2– and [Cr(OH)4]–. Centrifuging the solution will separate the solids from the complex ions in solution.
Fe3+(yellow-gold, aq) + 3 OH–(aq) → Fe(OH)3(brown, s)
Ni2+(green, aq) + 2 OH–(aq) → Ni(OH)2(green, s)
Mn2+(very pale pink, aq) + 2 OH–(aq) → Mn(OH)2(pale pink, s)
Zn2+(colorless, aq) + 4 OH–(aq) → [Zn(OH)4]2–(colorless, aq)
Cr3+(dark blue-violet, aq) + 4 OH–(aq) → [Cr(OH)4]– (blue-green, aq)
3–B: Oxidation of Cr
The [Cr(OH)4]– ion (+3 ox. state) is easily oxidized to the CrO42– anion (+6 ox. state) by H2O2 in a basic solution. The presence of a pale yellow solution indicates, but does not confirm, the presence of Cr3+.
2 [Cr(OH)4]–(aq) + 3 H2O2(aq) + 2 OH–(aq) → 2 CrO42–(aq) + 8 H2O(ℓ)
3–C: Dissolving the Hydroxide Solids
In this step the hydroxide solids dissolve by the reacting the OH– ions and the H+ ions (from HNO3). The resulting solution is divided into 3 portions or aliquots, to be used in subsequent steps to confirm the presence of Fe3+, Ni2+, and Mn2+.
Fe(OH)3(s) + 3 H+(aq) → Fe3+(aq) + 3 H2O(ℓ)
Ni(OH)2(s) + 2 H+(aq) → Ni2+(aq) + 2 H2O(ℓ)
Mn(OH)2(s) + 3 H+(aq) → Mn2+(aq) + 2 H2O(ℓ)
3–D: Confirmation of Fe3+
The addition of 0.2 M KSCN to the first aliquot produces a dark blood red solution. The formation of the blood red complex ion, [Fe(SCN)6]3–, confirms the presence of Fe3+. Ni2+ and Mn2+ do not react or interfere in this reaction.
Fe3+(aq) + 6 SCN–(aq) → [Fe(SCN)6]3–(aq)
3–E: Separation of [Ni(NH3)6]2+
The addition of 6 M NH3 (a weak base) to the second aliquot precipitates any Fe3+ or Mn2+ present as Fe(OH)3(s) and Mn(OH)2(s). Since the solid hinders your observation of the Ni confirmation test, discard any precipitate that forms. The ammonia complexes the Ni2+ ion to form [Ni(NH3)6]2+(aq), a pale sky blue solution. The presence of a pale sky blue solution indicates, but does not confirm, the presence of Ni2+.
Ni2+(aq) + 6 NH3(aq) → [Ni(NH3)6]2+(aq)
3–F: Confirmation of Ni2+
The addition of dimethylglyoxime, H2DMG, to the [Ni(NH3)6]2+ solution forms a strawberry red precipitate. The formation of the bright pink-red precipitate, Ni(HDMG)2(s), confirms the presence of the Ni2+.
[Ni(NH3)6]2+(aq) + 2 H2DMG → Ni(HDMG)2(s) + 2 NH4+(aq) + 4 NH3(aq)
3–G: Confirmation of Mn2+
A tiny amount of the powerful oxidizing agent, NaBiO3(s), is added to the third aliquot to oxidize the Mn2+ ion to the purple MnO4– ion (+7 ox. state). The formation of the permanganate ion, MnO4–, confirms the presence of Mn2+. If excess NaBiO3 is added, then the purple solution appears above a mustard brown solid.
2 Mn2+(aq) + 5 BiO3–(aq) + 14 H+(aq) → 2 MnO4–(aq) + 5 Bi3+(aq) + 7 H2O(ℓ)
3–H: Confirmation of Cr3+
The solution is heated in this step, to destroy any excess H2O2 still present. BaCl2(aq) is added to the solution to precipitate CrO4– as the pale yellow precipitate, BaCrO4(s). The appearance of the yellow barium chromate confirms the presence of Cr3+.
Ba2+(aq) + CrO42–(aq) → BaCrO4(s)
3–I: Dissociation of [Zn(OH)4]2–
The complex ion, [Zn(OH)4]2–, dissociates in acid to yield Zn2+ and OH– ions. The OH– ions further react with the H+ ions to produce H2O. The solution needs to be just weakly acidic for the next step.
[Zn(OH)4]2–(aq) + 4 H+(aq) → Zn2+(aq) + 4 H2O(ℓ)
3–J: Confirmation of Zn2+
The addition of K4Fe(CN)6(aq) to Zn2+(aq) produces a grayish white precipitate, ZnK2[Fe(CN)6](s). This precipitate may take a bluish tint if trace amounts of Fe3+ are present. The presence of a gray-white precipitate confirms the presence of Zn2+.
Zn2+(aq) + 2 K+(aq) + [Fe(CN)6]4–(aq) → ZnK2[Fe(CN)6](s)
Procedure
Always Wear Safety Goggles and Use Good Lab Practices
Chemical Alert:
Part I
Group 3 Known Solution
Dispose of all solutions and solids in their appropriate waste containers. DO NOT POUR DOWN THE DRAIN!
Record your observations and corresponding chemical formulas of the solids and/or ions for each step of the procedure.
Record appropriate inferences as you proceed, writing all of this on your lab notebook pages.
The formulas are found in the Background Information section online and in the flowchart below.
Part II
Start a Hot Water Bath with Distilled Water
3–A: Separation of Complex Ions
3–A1 Add 10 drops of the known cation solution to a small test tube.
Add 15 drops of 6 M NaOH to the test tube. Add 5 drops of water. Stir thoroughly.
3–A2 Centrifuge and decant the supernate into a clean test tube.
Keep the precipitate for step 3–C.
3–A3 If the decantate from the previous step is cloudy, add 3 additional drops of 6 M NaOH to the solution, stir, and centrifuge.
Keep the decantate for 3–B. Discard any precipitate formed. If not cloudy, go to 3–B.
3–B: Oxidation of Cr
3–B1 Add 2 drops of 3% H2O2 to the solution from 3–A.
Stir thoroughly and set the solution aside for step 3–H.
3–B2 The appearance of a yellow solution indicates, but does not confirm, the presence of Cr3+.
3–C: Dissolving the Hydroxide Solids
3–C1 Add 15 drops of 6 M HNO3 to the precipitate from 3–A and stir. Heat the mixture in a hot water bath for 2 minutes, stirring occasionally.
All of the solid should dissolve. If trace amounts of black solid remain, centrifuge.
Keep the solution and discard any residue.
3–C2 Add 15 drops of H2O to the solution. Stir and cool the solution.
Pour approximately the same volume of solution into 3 clean test tubes. Save these aliquots for 3–D, 3–E, and 3–G.
3–D: Confirmation of Fe3+
3–D1 Add 2 drops of 0.2 M KSCN to the first aliquot from 3–C2.
3–D2 Formation of a dark blood red solution confirms the presence of Fe3+. Show your results to your instructor for their initials. Discard the contents of the test tube in the waste container.
3–E: Confirmation of Ni2+
3–E1 Add 6 M NH3 to the second aliquot from 3–C2 until the solution is basic to litmus. Stir well after each addition of ammonia.
3–E2 Centrifuge and decant into a clean test tube for the next step.
Discard any precipitate that may have formed.
3–E3 Add 5 more drops of 6 M NH3 to the solution from above. If any additional solid forms, centrifuge. Save the solution for 3–F1 and discard any precipitate.
A pale sky blue solution indicates, but does not confirm, the presence of Ni2+.
3–F: Confirmation of Ni2+
3–F1 Add 4 drops of the dimethylglyoxime reagent (H2DMG) to the solution from 3–E3. Stir well and centrifuge.
3–F2 The formation of a strawberry red precipitate confirms the presence of Ni2+. If some precipitate remained from 3–E, then some brown solid may also form. Show your results to your instructor for their initials.
Discard the contents of the test tube in the waste container.
3–G: Confirmation of Mn2+
3–G1 Add 10 drops of H2O and 2 drops of 6 M HNO3 to the third aliquot from 3–C2 and stir.
3–G2 Add a very small portion of solid NaBiO3 (approx. the tip of a pencil eraser) to the solution. Stir thoroughly for 30 seconds. Centrifuge.
3–G3 The formation of a dark purple supernate confirms the presence of Mn2+. If excess solid was added, then some mustard yellow solid will also appear. The grape color may fade with time. Show your results to your instructor for their initials. Discard the contents of the test tube in the waste container.
3–H: Confirmation of Cr3+
3–H1 Place the solution from 3–B1 in a hot water bath and heat for 2 minutes. Cool the solution to room temperature.
3–H2 Add 5 drops of 0.2 M BaCl2 to the solution and stir well.
Centrifuge and save the decantate in a clean test tube for 3–I.
3–H3 The formation of a pale yellow precipitate confirms the presence of Cr3+. Show your results to your instructor for their initials. Discard the contents of the test tube in the waste container.
3–I: Dissociation of [Zn(OH)4]2–
3–I1 Add 6 M HCl dropwise slowly and with vigorous stirring to the decantate from 3–H2 until the solution is just acidic to litmus.
3–J: Confirmation of Zn2+
3–J1 Add 4 drops of 0.2 M K4Fe(CN)6 to the solution from 3–I.
Stir well and centrifuge.
3–J2 The appearance of a gray-white precipitate confirms the presence of Zn2+. If trace amounts of Fe3+ are present, the precipitate will take on a bluish tint.
Show your results to your instructor for their initials. Discard the contents of the test tube in the waste container.
Study Questions
1. What are the definitions of amphoteric and aliquot?
2. When NaOH is added in the first step to a known solution of Fe3+, Ni2+, Mn2+, Zn2+, Cr3+, what cations will NOT form a precipitate?
3. When NaOH is added in the first step to a known solution of Fe3+, Ni2+, Mn2+, Zn2+, Cr3+, what is/are the formulas of the precipitate?
4. When NH3 is added to a solution containing Ni2+, Mn2+, and Fe3+, what will happen? What is the formula of the product(s)?
5. When H2O2 is added to a solution containing both [Zn(OH)4]2– and [Cr(OH)4]–, what will happen? What is the formula of the product?
6. a. A solution is present in a test tube, but the test tube is not labeled. It could be Ni2+ or Mn2+. What reagent could you add to the solution to determine what it contains? Describe what will happen to the solution when the reagent is added if the solution contains Ni2+ and what will happen if it contains Mn2+.
b. A solution is present in a test tube, but the test tube is not labeled. It could be Ni2+ or Fe3+. What reagent could you add to the solution to determine what it contains? Describe what will happen to the solution when the reagent is added if the solution contains Ni2+ and what will happen if it contains Fe3+.
c. A solution is present in a test tube, but the test tube is not labeled. It could be [Zn(OH)4]2– and CrO42–. What reagent could you add to the solution to determine what it contains? Describe what will happen to the solution when the reagent is added if the solution contains [Zn(OH)4]2– and what will happen if it contains CrO42–.
d. A solution is present in a test tube, but the test tube is not labeled. It could be Zn2+ or Mn2+. What reagent could you add to the solution to determine what it contains? Describe what will happen to the solution when the reagent is added if the solution contains Zn2+ and what will happen if it contains Mn2+.
Activity Completed!