Chapter 1. Experiment 20
Various Reduction–Oxidation Reactions
Purpose of the Experiment
Observe various reduction–oxidation reactions and determine which species are being oxidized and reduced, and which are the reducing and oxidizing agents.
Background Required
This experiment will use basic laboratory techniques. The concepts of reduction– oxidation and oxidation numbers are used in this experiment.
Background Information
Reactions that involve the transfer of electrons from one species to another are called reduction–oxidation (redox) reactions. The species that loses electrons (has an increase in oxidation number) is oxidized. The species that gains electrons (has a decrease in oxidation number) is reduced. The electrons given up by the oxidized species are transferred to the species being reduced. Often we indicate which species causes the other to react. So the reducing agent is the species that causes the other species to be reduced. The oxidizing agent is the species that causes the other species to be oxidized.
As mentioned previously, redox reactions are also recognized by changes in oxidation numbers. In particular, it is easy to identify changes in oxidation numbers when an element appears in a chemical reaction as its element form and as a molecule or ion. Remember that any element in its elemental form has an oxidation number of zero. Also remember that any element in combination with another element will have a nonzero oxidation number.
Example
Problem
In the Copper Cycle experiment, Zn, a gray solid, was added to the blue Cu+2 solution. The blue color of the solution faded to colorless and some reddish-brown solid formed while the amount of gray solid diminished in amount.
What species was oxidized and what was reduced? What is the oxidizing agent and what is the reducing agent?
Solution
(1) By identifying the products, you can then determine the changes in oxidation numbers for the Cu and Zn. This will determine which species is being oxidized and which is reduced. This will also help in identifying the oxidizing and reducing agents.
a. The disappearance of the blue Cu+2 solution, indicates the Cu+2 ion is reacting to produce a different form. The reddish-brown solid produced is Cu(s). The half reaction for this observation is: Cu+2 (aq) → Cu(s).
To balance the half reaction 2 e– must be added to the reactant side. Therefore, this is a reduction half reaction or Cu+2 is being reduced.
Cu+2(aq) + 2e– → Cu(s)
Another way to determine the oxidized species is to look for a decrease or reduction of oxidation numbers. The oxidation number of Cu+2 is simply +2. The oxidation number of Cu(s) is zero (because it is in its elemental form). The oxidation number of Cu is reduced from +2 to 0; so Cu is being reduced.
b. The observation of the gray solid decreasing and forming a colorless solution indicates that the Zn(s) is reacting to form an aqueous ion (Zn+2). The half reaction for this observation is: Zn(s) → Zn+2(aq).
To balance the half reaction 2 e– must be added to the product side. This is an oxidation half reaction or Zn is being oxidized.
Zn(s) → Zn+2(aq) + 2 e–
c. The oxidation number of Zn is changing from a zero to a +2. An increase in oxidation number indicates that the Zn is being oxidized.
d. Since the Zn is providing electrons to the Cu+2 species, it is causing or forcing the Cu+2 to be reduced to Cu. So the Zn is the reducing agent. Likewise, the Cu+2 is taking electrons away from the Zn, causing the Zn to be oxidized, so the Cu+2 is the oxidizing agent.
In This Experiment
In this experiment, you will observe various reactions and determine if it is a redox reaction. If it is a redox reaction, you will also identify the species being reduced, species being oxidized, oxidizing agent and the reducing agent.
Procedure
Always Wear Safety Goggles and Use Good Lab Practices
Chemical Alert:
Part I
Reactions of Metals with HCl
1. Add a small piece of mossy Zn (the solid Zn) to a small test tube. Add approximately 2 mL of 10% HCl to the test tube. Observe the reaction and the speed of the reaction.
Look for changes in color at the surface of the solid, formation of a gas, and reduction in the mass of the solid.
2. Add a few pieces of the Mg turnings to a second small test tube. Add approximately 2 mL of 10% HCl to the test tube. Observe the reaction and the speed of the reaction. You may have to wait up to 5 minutes.
Verify the product(s) formed. H2(g) is a colorless gas. Zn2+(aq) and Mg2+(aq) are colorless solutions.
For the reaction in step 1, what is the oxidation number of Zn(s)? What is the oxidation number of Zn in Zn2+(aq)? What is the oxidation number of H in HCl(aq)? What is the oxidation number of H in H2(g)?
For the reaction in step 2, what is the oxidation number of Mg(s)? What is the oxidation number of Mg in Mg2+(aq)?
3. Any solid pieces of metal remaining should be placed in the solid waste beaker in the hood. Discard all of the solution down the drain with lots of running water.
Part II
Reaction of Cu2+ with Glucose
The reaction of Cu2+ with glucose is the basis of a test formerly used by diabetics to test blood sugars. Benedict’s reagent (Cu2+ in a basic solution) reacts as shown in the reaction below.
C6H12O6(aq) + 2 Cu2+(aq) + 6 H2O(ℓ) → Cu2O(s) + C6H12O7(aq) + 4 H3O+(aq)
4. Prepare a hot water bath by filling a 250 mL beaker approximately half full with water and placing it on a hot plate. Bring to a boil.
5. Add a small amount of glucose (enough to cover the tip of the spatula) to a small test tube. Add 2 mL of deionized water to the test tube and stir until the glucose dissolves.
6. Add 2 mL of Benedict’s reagent to the glucose solution. Observe the reaction. Heat the test tube in the hot water for 5 minutes. Observe the reaction.
Verify the product(s) formed. Copper(II) oxide (CuO) is a black solid. Copper(I) oxide (Cu2O) is a red solid. Cu2+(aq) is a blue solution. C6H12O6(aq) and C6H12O7(aq) are colorless solutions.
What is the oxidation number of Cu in Cu2+(aq)? What is the oxidation number of Cu in the product? Is the Cu oxidized or reduced? Is the C being oxidized or reduced?
7. Pour the contents of the test tube in the liquid waste container in the hood.
Part III
Reactions of MnO4–
8. Add 1 mL of 0.1 M K2C2O4 to a small test tube. Then add 10 drops of 6 M H2SO4 to the test tube. Stir to mix.
9. Add 1–2 drops of 0.1 M KMnO4 to the solution. Stir to mix. If there is no change, then heat in the hot water bath for 1 minute. Observe the reaction.
10. Add 3 mL of 0.1 M NaHSO3 to a second small test tube. Then add 1 mL of 1.0 M NaOH to the test tube. Stir to mix.
11. Add 1–2 drops of 0.1 KMnO4 to the solution. Stir to mix. If there is no change, then heat in the hot water bath for 1 minute. Observe the reaction. Pour reaction mixture in liquid waste container in hood.
Verify the product(s) formed. MnO4–(aq) is a dark purple solution. Mn2+(aq) is a very pale pink to nearly colorless solution. Manganese(IV) oxide (MnO2) is a black solid. CO2(g) is colorless gas. HSO3–(aq), SO42–(aq) and C2O42–(aq) are colorless solutions.
For the reaction in steps 8 and 9, what is the oxidation number of C in C2O42–(aq)? What is the oxidation number of C in the product? What is the oxidation number of Mn in MnO4–(aq)? What is the oxidation number of Mn in the product?
For the reaction in steps 10 and 11, what is the oxidation number of S in HSO3–(aq)? What is the oxidation of S in the product? What is the oxidation number of Mn in MnO4–(aq)? What is the oxidation number of Mn in the product?
Part IV
Reactions of ClO– + HCl (forms Cl2)
For this set of reactions, wear gloves and do not inhale the fumes from the test tubes!
Do not use Beral pipet to measure or dispense the CH2CI2! Use the dropper provided in the reagent bottle.
12. Add 5 drops of water to a clean, small test tube. Add 20 drops of dichloromethane (CH2Cl2) to the test tube.
Add 10 drops of 6 M HCl to the test tube.
Next, add 1 drop of bleach solution (5% NaClO) to the test tube.
Shake the test tube from side to side for about 1 minute.
Record all observations. Set the test tube aside.
13. Add 5 drops of 1.0 M NaBr, 20 drops of dichloromethane (CH2Cl2), and 10 drops of 6 M HCl to a clean, small test tube.
Record your observations.
14. Add 1 drop of bleach solution (5% NaClO) to the test tube.
Shake the test tube from side to side for about 1 minute.
Add 4 more drops of bleach solution.
Record all observations. Place test tube aside.
15. Add 5 drops of 1.0 M NaI, 20 drops of dichloromethane (CH2Cl2), and 10 drops of 6 M HCl to a clean, small test tube.
Record your observations.
16. Add 1 drop of bleach solution (5% NaClO) to the test tube.
Shake the test tube from side to side for about 1 minute.
Add 4 more drops of bleach solution.
Record all observations.
17. Add an additional 10 drops of bleach solution to the test tube.
Shake the test tube for 1 minute. Then let the solution sit in the test tube rack for an additional minute.
Record all observations.
Verify the product(s) formed. Cl– is colorless solution; Cl2 is yellow-green gas that dissolves in water and in CH2Cl2. Bromine, Br2, is an orange liquid that dissolves in CH2Cl2, Br–(aq) is a colorless solution. I2(aq) in the presence of excess I– forms a red-brown solution. I2(s) dissolves in CH2Cl2 to form a purple solution. I2(s) is dark purple (almost black).
In step 12, there is a reaction of ClO– with Cl– producing Cl2(g). This is a conproportionality reaction, where both of the half reactions form the same product. The overall reaction is given below.
2 H+(aq) + Cl–(aq) + ClO–(aq) → Cl2(g) + H2O(ℓ)
In steps 13 and 14, you once again form Cl2(g) first. Then the Cl2 reacted with the NaBr. What is the oxidation number of Cl in Cl2? What is the oxidation number of Cl in the product? What is the oxidation number of Br in NaBr? What is the oxidation number of Br in the product?
In steps 15 and 16, you once again form Cl2(g) first. Then the Cl2 reacted with the NaI. What is the oxidation number of Cl in Cl2? What is the oxidation number of Cl in the product? What is the oxidation number of I in NaI? What is the oxidation number of I in the product?
In step 17, the iodine product from step 16 further reacts with additional Cl2 in another reaction to produce IO3–(aq), a colorless solution. What is the oxidation number of I in IO3–?
Data Analysis & Determination
Determination
For each observed reaction, identify the products. Determine the oxidation numbers.
From the change in oxidation numbers, identify the species being reduced, the species being oxidized, the oxidizing agent, and the reducing agent.
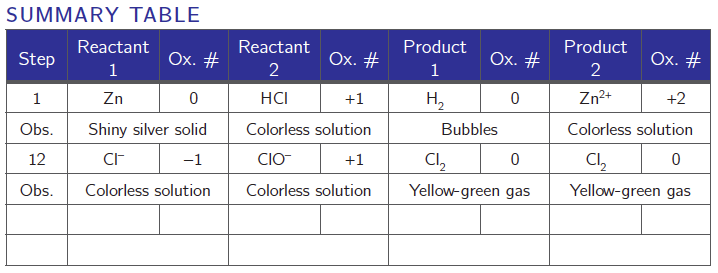
Produce a Summary Table similar to the one that follows with two of the reactions started. This will help you figure out what is being oxidized and reduced, and also what the oxidizing and reducing agents are.
Summary Table
Discussion
1. Summarize the results of this experiment.
2. What is the quality of the results? Do the results seem reasonable?
3. What were the errors or possible errors in the experiment?
4. How would the errors affect the results of the experiment?
Study Questions
Study Questions
1. Define reduction, oxidation, oxidizing agent, and reducing agent.
2. In a different rates of reaction experiment, the reaction being monitored was a redox reaction.
S2O8–2(aq) + 2 I–(aq) → 2 SO4–2(aq) + I2(aq)
Find the species being reduced, species being oxidized, oxidizing agent, and the reducing agent.
3. In a different rates of reaction experiment, the fast reaction was a redox reaction.
2 S2O3–2(aq) + I2(aq) → S4O6–2(aq) + 2 I–(aq)
Find the species being reduced, species being oxidized, oxidizing agent, and the reducing agent.
4. When steel wool (Fe(s)—a gray solid) is heated in the presence of oxygen (O2(g)—colorless gas), a red-brown solid forms (iron(III) oxide).
Write the balanced equation for this reaction.
Identify the species being reduced, species being oxidized, oxidizing agent, and the reducing agent.
5. Cu(s) does not react with 10% HCl. Compare the reactions of Zn + HCl, Cu + HCl, and Mg + HCl. Which reacted the most vigorously?
Which metal is the strongest reducing agent?
Which metal is the weakest reducing agent?
6. In the Copper Cycle experiment, the dissolution of copper solid by the hydrogen peroxide was labeled as a redox reaction.
Cu(s) + H2O2(aq) + 2 H+(aq) → Cu+2(aq) + 2 H2O(ℓ)
Find the species being reduced, species being oxidized, oxidizing agent, and the reducing agent.
7. When Cu(s) is heated in the presence of O2(g), the black solid, copper(II) oxide is formed.
Write the balanced redox reaction.
Find the species being reduced, species being oxidized, oxidizing agent, and the reducing agent.
Activity Completed!