Chapter 1. Experiment 4
Limiting Reagent Determination
Purpose of the Experiment
Determine which reactant is the limiting reagent in a synthesis experiment and from that, calculate the theoretical and percent yields.
Background Required
This experiment will use basic laboratory techniques of weighing, volume measurements, and vacuum filtration. The concepts of stoichiometry, limiting reagents, and theoretical, actual, and percent yield are used in this experiment.
Background Information
The product formed in this reaction is a coordination compound. A coordination compound is a chemical species that contains a metal ion surrounded by neutral molecules or anions (called ligands). In this experiment, neutral molecules, ethylenediamine, H2NCH2CH2NH2 or C2H8N2, will coordinate or bind to the Ni+2 ion to form a large complex cation, [Ni(C2H8N2)3]+2. To form a salt, chloride anions, Cl– supply the negative charge, resulting in trisethylenediaminenickel(II) chloride, [Ni(C2H8N2)3]Cl2. The ethylenediamine is often abbreviated as simply ‘en.’
NiCl2·6 H2O + 3 C2H8N2 → [Ni(C2H8N2)3]Cl2 + 6 H2O
NiCl2·6 H2O + 3 en → [Ni(en)3]Cl2 + 6 H2O
The theoretical yield is the amount of product that could be produced from the reactant if the reaction completely converts reactants into products. At the completion of the reaction, no reactant remains; it has been completely consumed. But if you start with two reactants, which reactant is consumed and which is present in excess? The limiting reagent is the reactant which is completely consumed or “used up” during the reaction. So calculations are based upon the moles of the limiting reagent.
In some reactions, physical observations of the reaction indicate which reactant is in excess. In this experiment, each of the reactants has different physical properties. The Ni+2 complexes are green to blue-violet in color; whereas the ethylenediamine is a weak base. If the filtrate is blue to blue-violet, then the NiCl2·6 H2O is in excess. However, if the filtrate is pink and is basic, then ethylenediamine is in excess.
In This Experiment
Each group will be assigned different starting amounts of the reactants. You will need to observe other groups’ end results. A diluted solution of ethylenediamine is used so that the volumes are easily measured.
The product is soluble in water, but insoluble in acetone. During the experiment, the volume of H2O is kept to a minimum. Acetone is added to the aqueous solution to make the acetone the solvent (present in the greater amount). Then the product will precipitate from solution as the chloride salt, [Ni(C2H8N2)3]Cl2.
Example
Problem
After 2.05 g of NiCl2·6 H2O reacted with 7.50 mL of the 4.0 M ethylenediamine, 1.87 g of the product, [Ni(en)3]Cl2 was obtained. The filtrate was pale pink and when tested with pH paper, the paper indicated a basic solution. What is the limiting reagent for the reaction, theoretical yield, and percent yield of [Ni(en)3]Cl2?
Solution
(1) Since the filtrate is basic and pink, this indicates that ethylenediamine is in excess. Therefore, the NiCl2·6 H2O is the Limiting Reagent. Calculate the theoretical yield of the product assuming the NiCl2·6 H2O is the Limiting Reagent.
Assuming NiCl2·6 H2O is Limiting Reagent:
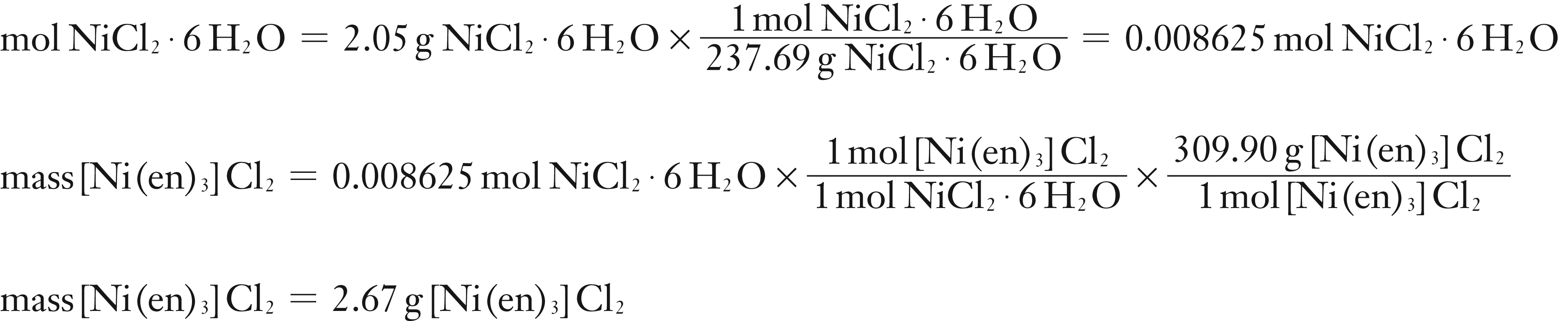
(2) To verify that NiCl2·6 H2O is the Limiting Reagent, a second calculation is performed. This time, assume en is the Limiting Reagent and calculate the theoretical yield.
If the NiCl2·6 H2O is indeed the Limiting Reagent (en is excess as indicated by the filtrate), then the theoretical yield from calculation (1) will be less than the theoretical yield from the calculation (2).
Assume H2NCH2CH2NH2 (en) is Limiting Reagent:
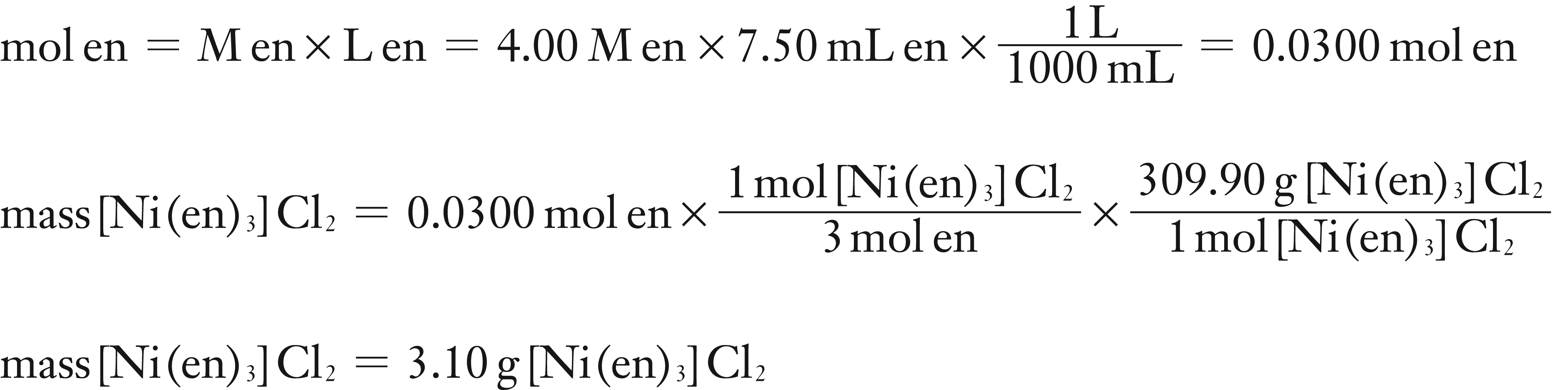
The smaller mass of product is from calculation (1). Therefore the theoretical yield is 2.67 g and NiCl2·6 H2O is the Limiting Reagent.
(3) Calculate the percent yield, using the theoretical yield (from the Limiting Reagent calculation) and the actual yield in the problem.

Procedure
Always Wear Safety Goggles and Use Good Lab Practices
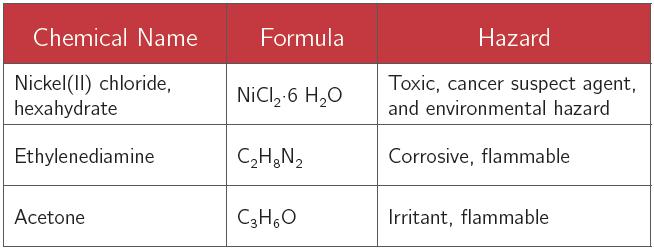
Chemical Alert:
Part I
Synthesizing [Ni(C2H8N2)3]Cl2
1. Your TA will assign one of the Trials to perform. This will give you the amounts of reagent to use in your experiment. Upon completion of your experiment, observe other groups’ results.
2. Tare the weight of a 150 mL beaker. Add enough NiCl2·6 H2O to the beaker until you obtain the mass required for your trial. Record the exact mass and observation of the solid.
3. With constant swirling after each drop, add deionized water dropwise to the solid until it just dissolves. Use the smallest volume of water possible, generally 2–3 mL. Record your observations.
4. Obtain the volume of 4.0 M C2H8N2 needed for your trial in a 10 mL graduated cylinder. Record the exact volume and observation of the solution.
5. Using a Beral pipet, very slowly add the C2H8N2 solution dropwise to the beaker with constant stirring. It should take approximately 5 minutes to add all of the solution. Record all observations during the reaction, especially all of the color changes observed during the addition of the C2H8N2.
Part II
Precipitating and Recovering [Ni(C2H8N2)3]Cl2(s)
6. Obtain about 25 mL of acetone in a clean 50 mL beaker (to be used in several steps). Label a clean, dry 10 mL graduated cylinder for use with the acetone.
7. Pour 5 mL of acetone into the 10 mL graduated cylinder. Add this aliquot (portion) to the reaction beaker, stirring well after the addition.
Add a second 5 mL aliquot of acetone to the beaker, stirring well.
Add a third 5 mL aliquot of acetone to the beaker, stirring well.
8. Prepare an ice bath in a 400 mL beaker. The beaker should only be half full.
9. Place the reaction beaker in the ice bath and let it sit undisturbed for 15 minutes.
Record all observations, especially in regard to color.
10. Set up a vacuum filtration apparatus.
If needed, refer to the illustrated figure in the previous experiment.
Place a piece of filter paper in the Büchner funnel. Moisten the filter paper with a minimal amount of water and begin filtration.
Stir the reaction mixture to suspend the product. Pour the entire mixture all at once into the funnel. Use a spatula to scrape any remaining solid from the beaker into the funnel. Continue to pull air through the sample to dry the sample for 3 minutes.
11. Rinse with another 5 mL of acetone. Continue to pull air through the sample until the solid appears dry (about 5 minutes). Disconnect the hose and then turn off the aspirator.
12. Using the weighing by difference technique, weigh a clean watch glass and record its mass. Then scrape the entire solid off the filter paper onto the watch glass and reweigh.
Record the mass of solid + watch glass. Record your observation of the solid.
Part III
Determining the Excess Reagent
13. Record your observation of the filtrate in the filter flask.
If the filtrate is a dark blue to blue-violet solution, then the dissolved NiCl2·6 H2O is present and is the excess reagent.
If the filtrate is a pink to pale lavender solution, then the ethylenediamine solution is present and is the excess reagent.
14. Tear off a piece of pH paper and place on a clean watch glass. Stir the filtrate with a clean stirring rod and then touch it to the pH paper. Record your observation.
Compare the color of the wet pH paper to the color chart on the dispenser. If the color matches the pHs in the 9–13 range, the solution is basic. Remember that ethylenediamine is a base. The filtrate will be basic if the ethylenediamine is present and is the excess reagent.
15. Discard the filtrate in the appropriate waste bottle in the hood. Place the [Ni(en)3]Cl2 solid in the appropriate waste container in the hood.
Data Analysis
Calculations & Determinations
Based on your observations in Part III, which reagent is present in excess? Which reagent is the Limiting Reagent (completely used up)?
Calculate the theoretical yield of [Ni(en)3]Cl2 upon your assumption of the Limiting Reagent.
Calculate the theoretical yield of [Ni(en)3]Cl2 using the excess reagent.
Did your observations agree with your calculations?
Calculate the percent yield of the [Ni(en)3]Cl2.
Discussion
1. Summarize the results of this experiment.
2. What is the quality of the results? Do the results seem reasonable?
3. What were the errors or possible errors in the experiment?
4. How would the errors affect the results of the experiment?
Study Questions
Study Questions
1. a. What will you observe after the reaction is complete if NiCl2·6 H2O is present in excess?
b. What will you observe after the reaction is complete if C2H8N2 is present in excess?
2. Define limiting reagent, excess reagent, and aliquot.
3. Nick, Ethyl, and Amy reacted 3.60 g of NiCl2·6 H2O with 8.50 mL of 4.0 M C2H8N2 and obtained 2.83 g of [Ni(en)3]Cl2. The filtrate was dark blue.
a. What is the Limiting Reagent?
b. What is their theoretical yield?
c. What is their actual yield?
d. What is their percent yield?
4. Explain what effect each of the following will have on the actual yield of the [Ni(en)3]Cl2.
a. Student used 10 mL of water to dissolve the NiCl2·6 H2O.
b. Student used water to rinse the [Ni(en)3]Cl2 product.
c. Student used 12.0 mL of 4.0 M C2H8N2 but recorded the volume as 8.0 mL and used en as the limiting reagent in the calculations.
5. Was the color of the [Ni(en)3]Cl2 product the same when NiCl2·6 H2O was the limiting reagent as compared to when C2H8N2 was the limiting reagent?
6. a. What was done during filtration to help reduce errors that would lead to a percent yield greater than 100%? Explain how this reduced the error.
b. What was done during filtration to help reduce errors that would lead to a percent yield less than 100%? Explain how this reduced the error.
Activity Completed!