Chapter 1. Experiment 6
Vitamin C in Fruit Juices
Purpose of the Experiment
Estimate how much Vitamin C (ascorbic acid) is present in a fruit juice.
Background Required
This experiment will use basic laboratory titration techniques. The concepts of oxidation–reduction reactions and stoichiometry are used in this experiment
Background Information
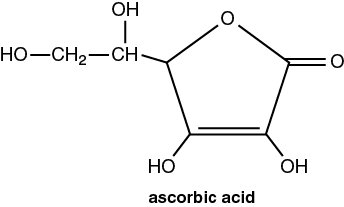
Vitamins are a class of essential compounds that can’t be synthesized by the body; yet small amounts must be ingested for continued good health. Humans require 13 different vitamins. Humans and most primates cannot synthesize their own Vitamin C, ascorbic acid (C6H8O6). The revised Daily Recommended Intake (DRI) of Vitamin C for adults is 90 mg per day. However in the 2016 Vitamin C Fact Sheet for Consumers, the National Institute of Health states that the amount of vitamin C needed depends on your age. For infants up to 6 months, the recommended amount is 40 mg/day and the highest amount is 120 mg per day for breastfeeding women. Scientists studying Vitamin C have found results in cancer prevention & treatment, a lower risk for cardiovascular disease, and when combined with other nutrients might slow the progression of macular degeneration and cataract formation in older adults’ eyes.
This titration uses an oxidation–reduction reaction between the analyte (ascorbic acid) and the titrant. This reaction is more specific in reacting only with the ascorbic acid. The titrant for this reaction is 2,6-dichloroindophenol (C12H7O2NCl2, abbrev. DCIP). Since this compound is deep blue initially, colorless after it reacts, and red in acidic solutions, it acts as its own indicator. In the buret, the DCIP will be deep blue, but after it reacts with the analyte (ascorbic acid) in the flask, it will be colorless. When there is no more ascorbic acid, the end point will be signaled by the excess DCIP turning pink-red in the acidic analyte solution.
The DCIP solution is not stable, so it must have its concentration determined before you use it. The method of determining the concentration of the titrant by using a known amount of a standard compound is called standardization. By determining how many moles of titrant (DCIP) were needed to react with an aliquot (a portion) of the standard (ascorbic acid), one can determine the molarity of the DCIP. Then, a second set of titrations will be performed with the DCIP and the analyte, ascorbic acid in the fruit juice. This reaction must take place in acidic solutions. In the first part, a pH 3 buffer is added. In second part with the fruit juice, metaphosphoric acid, (HPO3)n is added.
C6H8O6(aq) + C12H7O2NCl2(aq) → C6H6O6(aq) + C12H9O2NCl2(aq)
In This Experiment
In this experiment, a solution of pure ascorbic acid (standard) will be prepared. The first set of titrations with the standard ascorbic acid will determine the concentration of the DCIP (titrant). The second set of titrations will determine the amount of ascorbic acid, Vitamin C, in the fruit juice. Finally, you will determine how much fruit juice needs to be consumed to satisfy the DRI of Vitamin C for adults. Review the good lab titrating techniques from the previous experiment.
Example
Problem
A standard solution of ascorbic acid was prepared by dissolving 0.0470 g of ascorbic acid in a 100 mL (0.100 L) volumetric flask. It required 24.08 mL of DCIP to titrate 10.0 mL of the standard ascorbic acid to its end point.
What is the molarity of the standard ascorbic acid solution?
What is the molarity of the DCIP solution?
Solution
(1) Using the mass of C6H8O6, molar mass (molecular weight) and the total volume of solution, calculate the M of the standard C6H8O6 solution.
(2) Using the M and mL of C6H8O6 and stoichiometry to find moles of DCIP. Then divide moles of DCIP by the L of DCIP to find the M of DCIP.
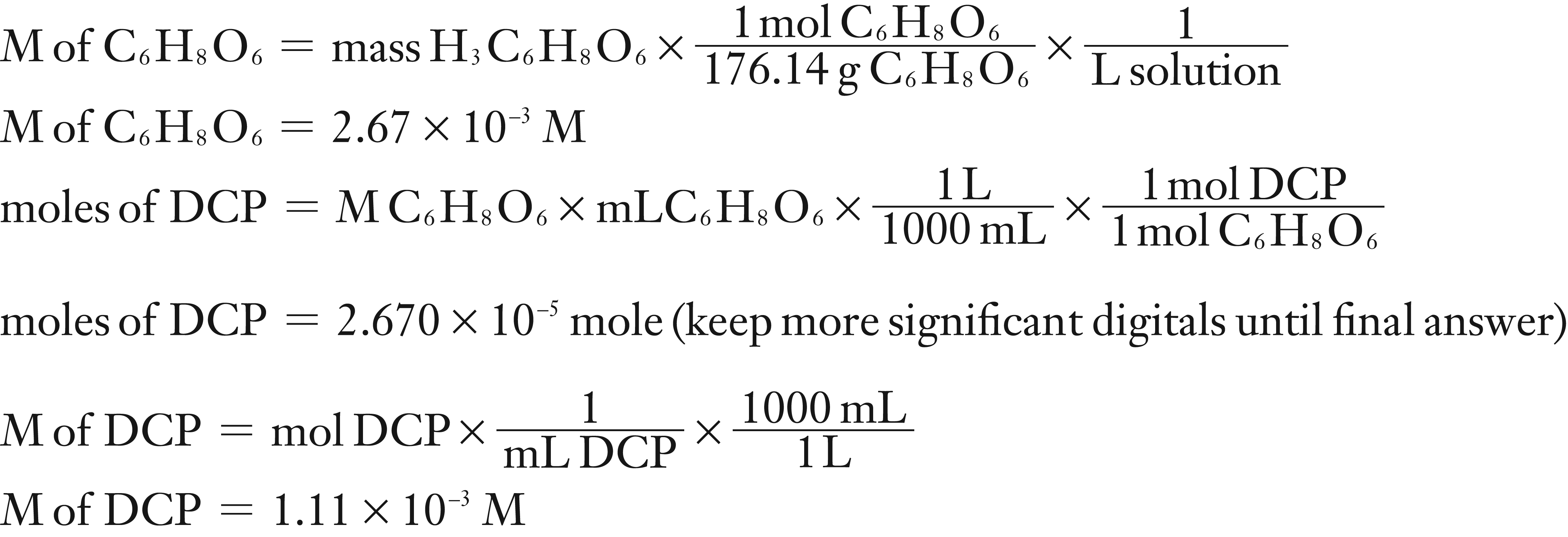
Problem
It required 16.24 mL of DCIP to titrate 5.00 mL of lime juice to its end point. Calculate how many mg of ascorbic acid is present in the juice.
How many mL of juice is needed to satisfy the DRI of Vitamin C?
Solution
(1) Using the M and mL of DCIP, stoichiometry, and the molar mass (molecular weight) of C6H8O6, calculate the mg of C6H8O6.
(2) Divide the DRI (90 mg of C6H8O6) by mg of C6H8O6 per mL of juice.

Procedure
Always Wear Safety Goggles and Use Good Lab Practices
Chemical Alert:
Part I
Preparing Standard C6H8O6 Solution
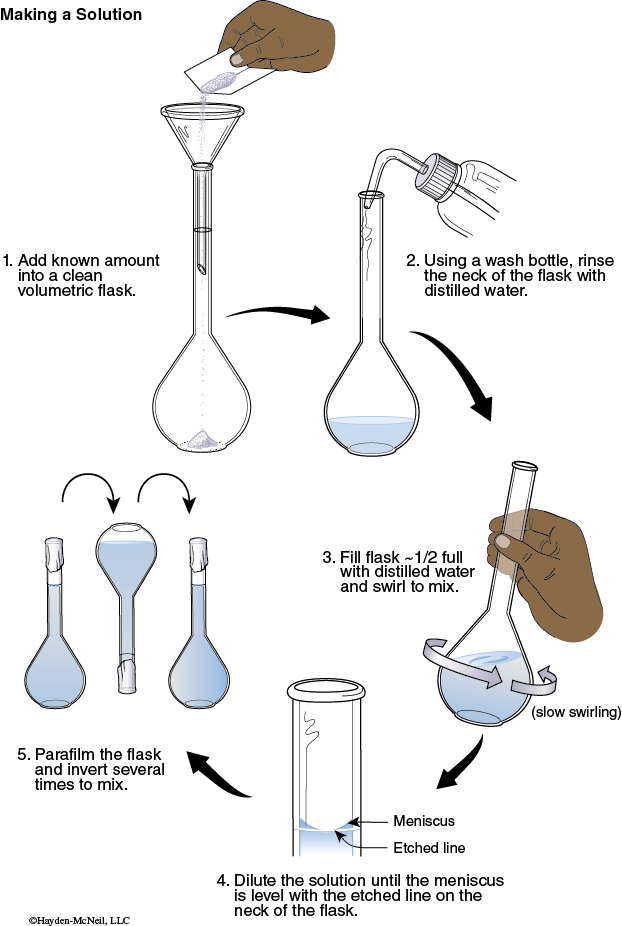
1. Using the tare feature on the balance, tare the weight of the weighing paper to zero. Add enough C6H8O6 until you obtain a mass of C6H8O6 that is between 0.025–0.035 g. Record this exact mass. Transfer the C6H8O6 to a 100 mL volumetric flask.
2. Use a stream of deionized water from a wash bottle to rinse down into the solution any solid clinging to the inside neck of the flask.
3. Fill the flask about half full with deionized water. Swirl the flask until all of the solid dissolves.
4. Add enough deionized water to fill the flask to the calibration mark. Seal the mouth of the flask with Parafilm.
5. Invert the flask several times to mix. This is your standard C6H8O6 solution.
Part II
Standardizing the DCIP Solution
6. Transfer exactly 10.00 mL of C6H8O6 standard solution to a clean 250 mL Erlenmeyer flask. Add 20 mL of deionized water and 10 mL of pH 3 buffer to the flask. Swirl to mix.
7. Rinse the buret with some of the DCIP solution and then set up the buret for titration. Fill the buret with the DCIP (titrant) and then eliminate any bubbles in the tip of the buret. Drain some of the solution out. Record the initial buret reading.
8. Titrate the C6H8O6 standard solution with the DCIP to the end point.
Pink color that stays for 30 seconds.
Record the final buret reading. Refill the buret.
9. Prepare two more solutions as in step 6. Titrate each solution to the end point. Record your initial and final buret readings for each titration.
10. Pour all solutions down the drain with lots of running water. Rinse out the 250 mL Erlenmeyer flasks.
Part III
Titrating the Fruit Juice
11. Obtain about 20 mL of the fruit juice (analyte) in a beaker.
12. Transfer exactly a 5.00 mL aliquot of the juice to a clean 250 mL Erlenmeyer flask. Add 10 mL of 3% (HPO3)n to the flask. Swirl to mix.
13. Titrate the fruit juice with the DCIP to the end point. Record the initial and final buret readings. Refill the buret.
14. Prepare two more solutions as in step 12. Titrate each solution to the end point. Record your initial and final buret readings.
15. Pour all solutions down the drain with lots of running water. Drain out all the remaining titrant. Rinse out buret with water, allowing water to drain through the tip.
Calculations
Calculate the molarity of your standard C6H8O6 solution.
For each titration in Part II (Standardizing the DCIP Solution), calculate the molarity of the DCIP. Calculate the average DCIP molarity.
For each titration in Part III (Titrating the Fruit Juice), calculate the mg of Vitamin C (C6H8O6) present. Calculate the average mg of Vitamin C present.
Determine how many mL of juice are needed to meet the adult DRI of Vitamin C.
Discussion
1. Summarize the results of this experiment.
2. What is the quality of the results? Do the results seem reasonable?
3. What were the errors or possible errors in the experiment?
4. How would the errors affect the results of the experiment?
Study Questions
Study Questions
1. For each chemical, match it to its function name and where you will find it in the experiment.
2. How will you recognize the end point of the titration?
3. Vida and Min dissolved 0.0521 g of C6H8O6 in a 100 mL volumetric flask with deionized water. A 10.0 mL aliquot of this standard C6H8O6 solution required 23.7 mL of DCIP to reach the end point. A 5.00 mL aliquot of orange juice required 14.4 mL of the same DCIP solution to reach the end point. a. What is the molarity of the standard ascorbic acid solution? b. What is the molarity of the DCIP solution? c. How many mg of C6H8O6 are present in the juice sample? d. How many mL of orange juice are needed to satisfy the adult DRI requirement for Vitamin C?
4. Titrations are a type of stoichiometry calculation. In this experiment what is the mole ratio between ascorbic acid and DCIP?
5. How much juice would have to be consumed to satisfy the NIH’s recommendation of 200 mg per day? How much juice would have to be consumed to satisfy the 2002 study’s minimum recommendation of 6,000 mg per day?
6. How would the following errors affect the calculations indicated? Will it make the values too large or too small or no effect? Explain your answers. a. Overfilling the volumetric flask in determining molarity of standard ascorbic acid. b. Overtitrating the end point in determining the molarity of the DCIP. c. Transferring 10.00 mL of juice, but used 5.00 mL in calculating the mg of C6H8O6 present in the juice. d. Overtitrating the end point in determining the mg of C6H8O6 in the fruit juice sample. e. Transferring 10.00 mL of juice, but used 5.00 mL in calculating the mL required to satisfy the DRI of Vitamin C.
7. By examining how close the titrations are to each other, you can look at the precision (reproducibility) of your titrations. This is an indication of how well you performed the titrations. Look at your titrations. Did you have good precision? If so, what did you do to help with the precision? If not, what could you do in the future to improve your precision?
8. Vitamin C is found in many fruits and vegetables. Vitamin C is also oxidized by oxygen in the air. Which would have more Vitamin C present, fresh spinach or spinach that has been boiled for 10 minutes? What effect would peeling a tomato and letting it sit have on the amount of Vitamin C present?
Activity Completed!