Chapter 1. Experiment 8
Determining % Composition of H2O2
Purpose of the Experiment
Determine the % mass composition of H2O2 by measuring the production of O2.
Background Required
This experiment will use laboratory techniques of gas production. Also used are techniques of volume measurements. The concepts of Ideal Gas Law, Dalton’s Law of Partial Pressures, stoichiometry, molarity, and % composition are used in this experiment.
Background Information
Hydrogen peroxide commonly comes in 2 different concentrations, 30% H2O2 and 3% H2O2. You will determine which concentration is present initially in your sample of H2O2. Oxygen gas (O2) is produced in the decomposition of hydrogen peroxide (H2O2). By measuring how much O2 is produced, you can use Dalton’s Law of Partial Pressures, Ideal Gas Law, and stoichiometry to determine how much H2O2 was present originally.
2 H2O2(aq) → O2(g) + 2 H2O(ℓ)
In This Experiment
Yeast is used to catalyze the decomposition of the hydrogen peroxide. As the oxygen gas is produced, it displaces water in a graduated cylinder. Since water is present, there is both oxygen gas and water vapor present. Dalton’s Law of Partial Pressures allows us to subtract out the water vapor to find the partial pressure due to the O2. Then using the partial pressure and volume of O2, the Ideal Gas Law is rearranged to find moles of O2. Next, by using stoichiometry and the balanced equation above, the moles of H2O2 can be determined. Finally, the molarity of the H2O2 is determined and then converted to mass percent.
Example
Problem
Determine if the concentration of H2O2 solution is 30% or 3% by mass. When 8.03 mL of the H2O2 solution reacted with the yeast at 23.7°C, 85.89 mL of gas was produced. The barometric pressure was 752.4 torr. The vapor pressure of H2O at 24°C is 22.4 torr.
Solution
(1) Using Dalton’s Law of Partial Pressures find the partial pressure of O2 in the collected gas.
(1) Find PO2 using Dalton’s Law of Partial Pressures
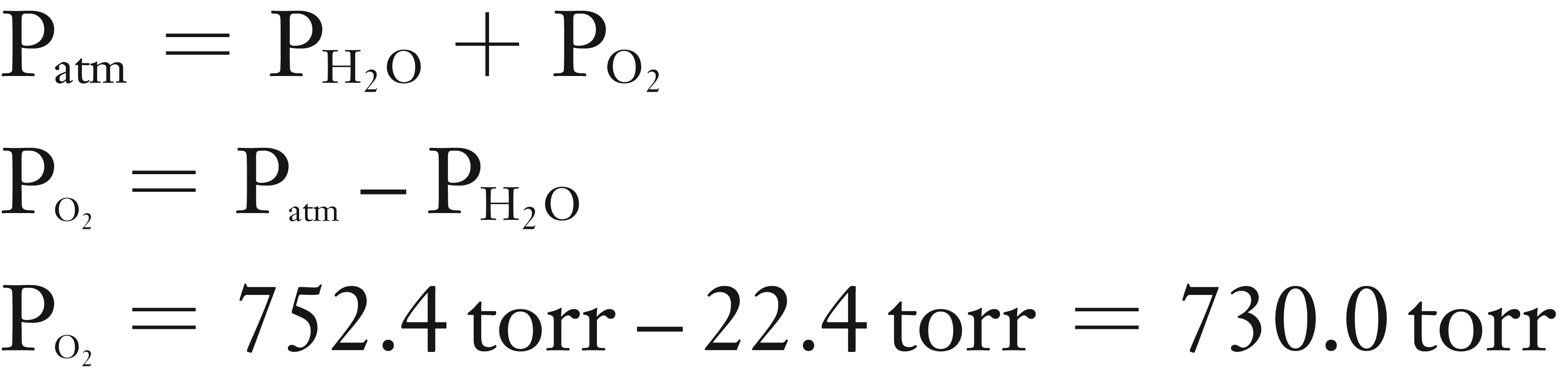
Solution
(2) Using the Ideal Gas Law, find moles of O2
(2) Find moles of O2 using PV = nRT
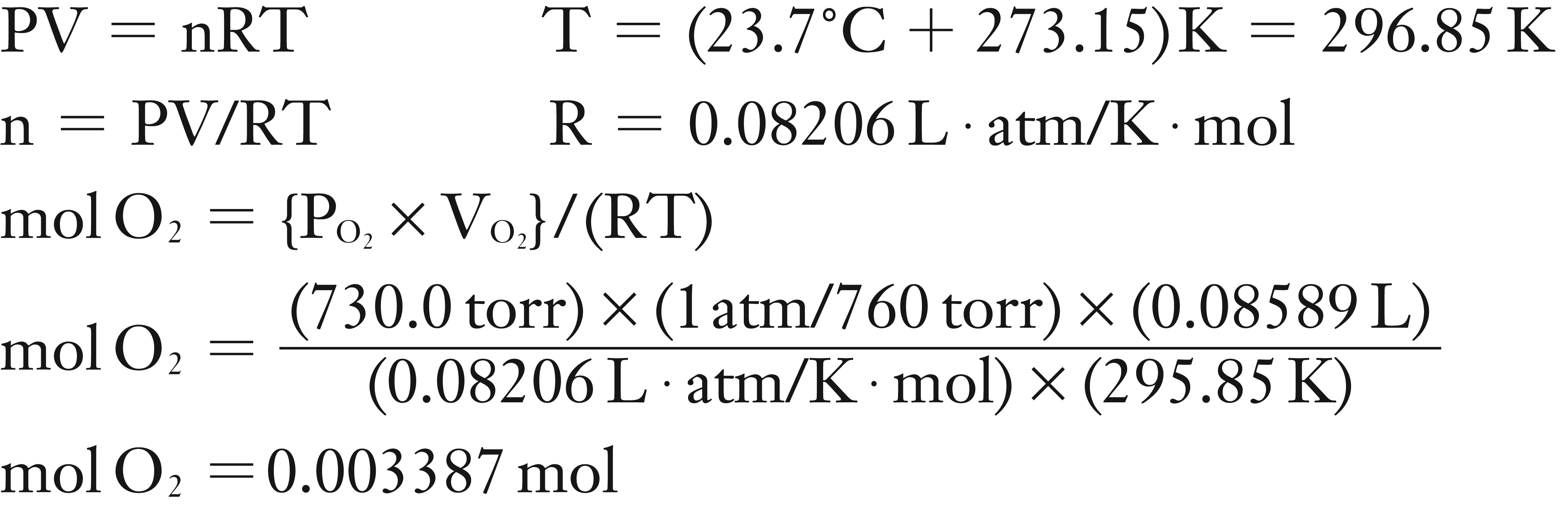
Solution
(3) Using stoichiometry find the moles of H2O2 that produced this O2
(3) Find moles of H2O2 using stoichiometry and then M of H2O2
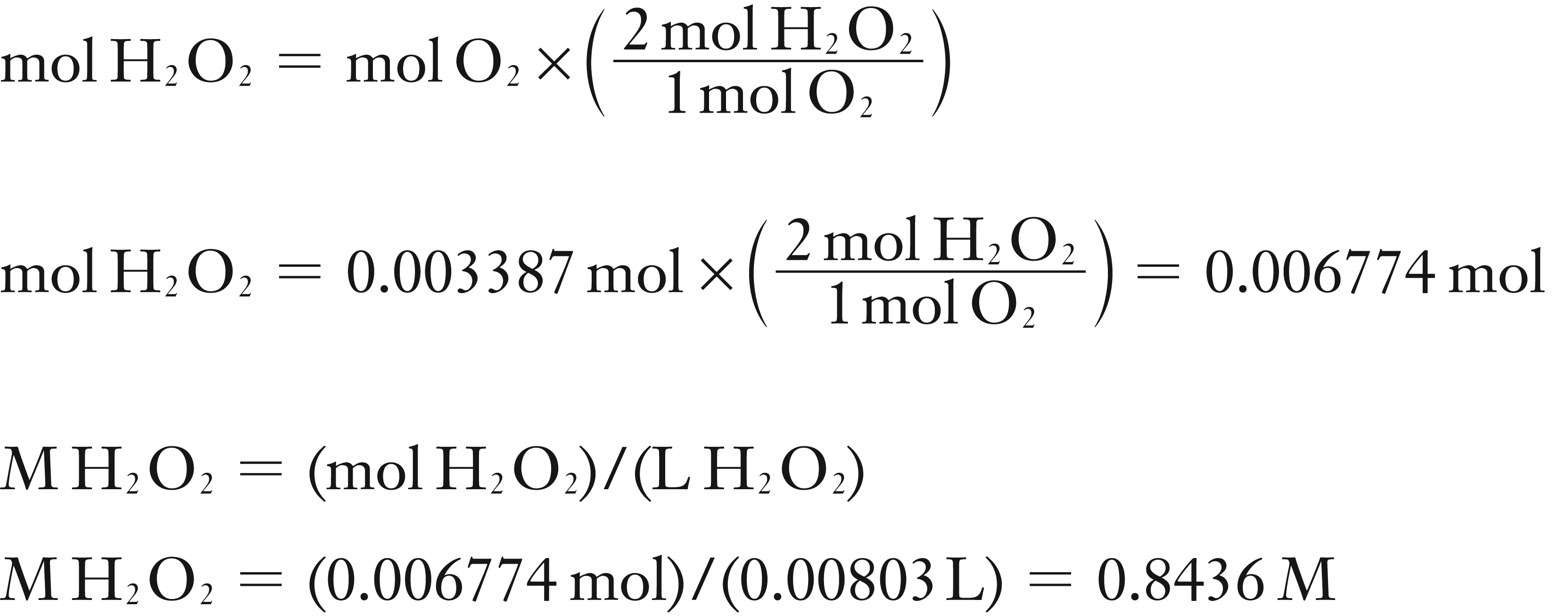
Solution
(4) Convert M to % by mass using molar mass of H2O2 and assuming the density of the H2O2 is the same as water, 1 g/mL.
(4) Find % mass using Molar Mass of H2O2 and Density of H2O2 solution
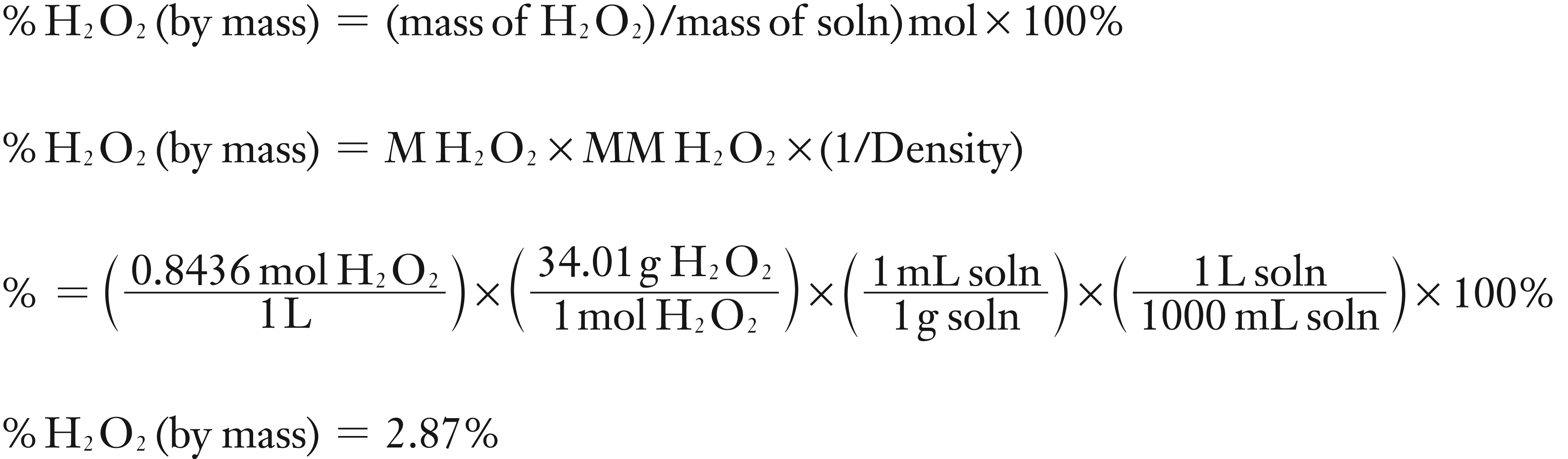
The experimental value of 2.87% H2O2 solution is closer to 3% than 30%. So the solution is 3.00% H2O2.
Procedure
Always Wear Safety Goggles and Use Good Lab Practices
Chemical Alert:
Part I
Setting up the Gas Collection Apparatus
1. Obtain ~30 mL of the H2O2 solution in a clean 50 mL beaker.
2. Obtain a small scoop of yeast on a clean, dry watch glass.
3. Fill a 1000 mL beaker with approximately 500 mL of water and place on the foot of a ring stand.
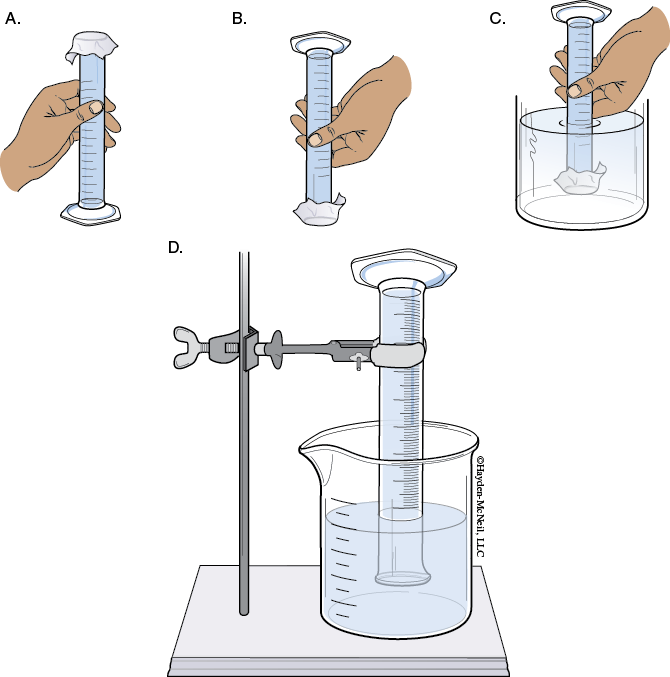
4. Next fill a 100 mL graduated cylinder as full as possible. Seal the top of the graduated cylinder with Parafilm.
Invert the graduated cylinder and place the mouth of the cylinder under the water level in the beaker. While still under the water level, pull back the Parafilm halfway.
Clamp the cylinder onto the ring stand so the mouth remains under the water level. Next add enough water to the beaker to have approximately 800 mL.
Obtain a large test tube and clamp it to the ring stand as shown in the illustration below. Make sure that the graduated cylinder and test tube are clamped into a vertical position.
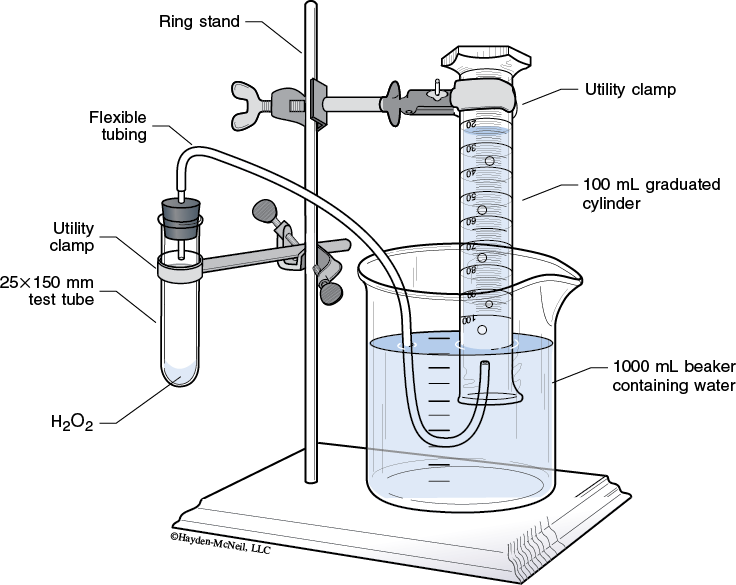
5. Some air may have escaped into the graduated cylinder. Read and record volume of the gas. (If no gas is present, the initial volume is zero.)
6. Measure between 7–9 mL of the H2O2 solution into a 10 mL graduated cylinder. Record the EXACT volume. Pour into the test tube. Loosely stopper the test tube.
Use crucible tongs to guide the hose into the mouth of the inverted graduated cylinder. The Parafilm holds the hose in the mouth of the cylinder.
Part II
Reacting the H2O2
7. Lift the stopper and using the spatula, scrape the yeast off the watch glass until you have a layer of yeast in the bottom of the test tube. QUICKLY restopper the test tube. The reaction with the H2O2 starts nearly immediately. If the yeast is floating on the surface of the solution, give the test tube a little shake.
8. The bubbles of O2 produced should be collecting in the graduated cylinder, displacing the water out of the cylinder. The reaction may take ~20 minutes.
9. When the reaction is complete (no more bubbles forming), align the water level in the graduated cylinder with the water level in the beaker. To do this, raise or lower the graduated cylinder (or add/remove water from the water bath) until the two water levels are equal.
Now the pressure in the graduated cylinder is equal to the atmospheric pressure. Read and record the final gas volume.
10. Obtain a LabQuest and temperature probe. Turn on and connect the temperature probe. Place the probe in the beaker of water. Record the temperature of the water.
Use this temperature to find the H2O vapor pressure in Table 8-1. This temperature will also be used as the temperature of the gas. Return LabQuest and temperature probe.
11. Pour the contents of the test tube down the drain with lots of water. Rinse the test tube and reclamp into place. Pull the graduated cylinder out of the water. Pour water out of the beaker until you have about 500 mL remaining.
12. Repeat steps 4–11 for two additional trials.
13. Pour all liquids down the drain. Any excess yeast can go into the trash can. Return 1000 mL beaker, large test tube, and crucible tongs to the back of the lab.
14. Your TA will give you today’s barometric pressure (Patm).
Calculations
For each trial, calculate the partial pressure of O2, moles of O2, moles of H2O2, and Molarity of H2O2.
Conversion factors and constants: R = 0.0821 L·atm/K·mol; 760 torr = 1 atm
Find the average H2O2 molarity from the three trials.
Next, convert the average Molarity to % composition (by mass).
Assume that the density of the H2O2 solution is 1.0 g/mL.
Is the solution 30% or 3% H2O2?
Discussion
1. Summarize the results of this experiment.
2. What is the quality of the results? Do the results seem reasonable?
3. What were the errors or possible errors in the experiment?
4. How would the errors affect the results of the experiment?
Study Questions
1. What is the purpose of the yeast in this experiment?
2. a. What is the partial pressure of O2 if the barometric pressure is 745.2 torr and the water temperature was 28°C?
b. What is the % by mass composition of H2O2 if the Molarity of the H2O2 is 4.72 M? Assume the density of the H2O2 is 1.00 g/mL.
c. How many moles of O2 are present if 79.3 mL of O2 has a partial pressure of 721.8 torr at 23.7°C?
d. What is the Molarity of an H2O2 solution if 0.00362 moles of O2 was produced by 7.82 mL of H2O2 solution?
3. Why do we need to subtract the water vapor pressure from the barometric pressure?
4. How would the following errors affect the calculation of Molarity of H2O2? Explain your answers.
a. The rubber stopper with the attached hose was not fitted tightly into the test tube after the yeast was added. (Some gas escaped out of the test tube.)
b. The initial volume of gas in the graduated cylinder was recorded as zero instead of 3.5 mL.
5. Perry read the final volume of gas as 88.6 mL before aligning the water levels. His partner Ida read the final volume of gas as 91.3 mL AFTER aligning water levels. Will Perry’s calculation of percent by mass be higher or lower than Ida’s? Explain your reasoning.
6. How many mL of O2 would be produced by the reaction of 8.03 mL of 30.0% H2O2? The barometric pressure (Patm) was 752.4 torr and the temperature was 24.0°C. (Hint: Work this problem in reverse. Convert % H2O2 by mass to M of H2O2, then moles of H2O2, then moles of O2, then L of O2.)
Activity Completed!