Chapter 1. Experiment 9
Determination of Aspirin by Spectrophotometric Analysis
Purpose of the Experiment
Determine the amount of aspirin present in an aspirin tablet by spectrophotometric analysis.
Background Required
You should be familiar with basic laboratory techniques for volume measurements and volumetric glassware. You should also be familiar with graphing, determining the slope of a line, and calculating solution stoichiometry problems.
Background Information
One technique used to determine how much of a chemical species is present in a sample is spectroscopy. If a chemical species forms a colored solution, there is a direct correlation between the concentration of the solution and the intensity the color. Using this relationship, the concentration of solution is determined by measuring the intensity of the color with a spectrophotometer. Light shines through the sample solution, and a detector measures the intensity of the light after it passes through the sample. As the intensity of the color increases, more light will be absorbed by the solution, hence less light will pass through to the detector.
The chemical species that we are investigating is aspirin, or acetylsalicylic acid. Commercially, aspirin is available in 3 strengths, extra strength (500 mg), regular strength (325 mg), and low dosage (81 mg). In water, aspirin is hydrolyzed to produce salicylic acid and acetic acid. But if the reaction occurs in a strongly basic solution, the sodium salts (sodium salicylate and sodium acetate) are formed.
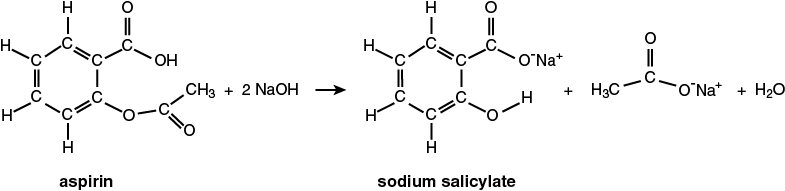
Since aqueous solutions of the salicylate ion are colorless, a reagent must be added that reacts with the salicylate ion to create a colored chemical species. For this experiment, the “color producing” reagent is iron(III) chloride, FeCl3. In water, water molecules coordinate to the Fe+3 cation to form [Fe(H2O)6]+3 ion. The light yellow ion, [Fe(H2O)6]+3, reacts with the colorless salicylate ion to form the purple [Fe(salicylate)(H2O)4]+ ion as shown in the second reaction.
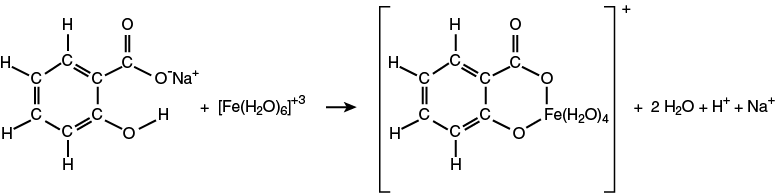
The intensity of the purple color of the solution is directly proportional to concentration of the salicylate ion present. Therefore, the more salicylate ion present in the solution (from the hydrolysis reaction of aspirin) the greater the absorption of light. You will determine how much aspirin was present in an unknown aspirin tablet.
In This Experiment
To determine the concentration of an unknown solution, you must compare it to a set of solutions with known concentrations in a calibration plot. For the calibration plot, you will prepare several dilute solutions of sodium salicylate of known molarities and add FeCl3 to create colored solutions. Next, the amount of light these colored solutions absorb is measured using the spectrophotometer. Graphing the absorbances of the salicylate solutions versus their concentrations creates a standard calibration plot. When the concentration is zero, no light will be absorbed, so the line of the data must go through the origin, (0,0). The equation of the line in the plot, shown in Equation 1, is called Beer’s law.
An unknown aspirin tablet will be dissolved in a basic solution to create a sodium salicylate solution. Then an aliquot (a small sample) of this solution will be diluted by adding the FeCl3 to form a dilute colored solution. Its absorbance will be measured using the spectrophotometer. By using the equation of the line from the calibration plot, you will determine the concentration of salicylate ion in the dilute aspirin solution. Finally, you will determine the amount of aspirin in the original sample and decide if it was a 500 mg, 325 mg or 81 mg aspirin tablet.
You will be working with very dilute concentrations, therefore very careful and exact work is required. Care must be taken to properly wash and rinse glassware before using. A trace of contaminants can ruin the experiment!
Example
Problem
A 0.020 g sample of sodium salicylate (molar mass = 160.11 g/mol) was dissolved in 100 mL of water. Next a 5.0 mL aliquot of this concentrated sodium salicylate solution was diluted to 100 mL. What is the molarity of the new dilute sodium salicylate solution?
Solution
(1) Calculate the molarity of the concentrated sodium salicylate solution using mass, molar mass (160.11 g/mol), and volume.
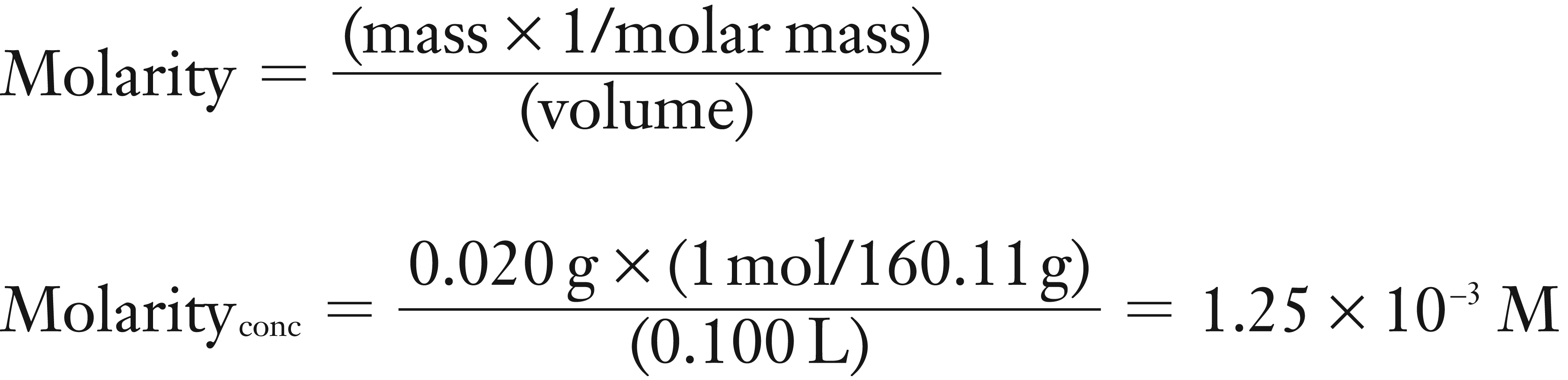
(2) This is a dilution problem. Use Equation 2 to solve for the missing variable, Molaritydilute using the Mconc, Volconc, and Vdilute.
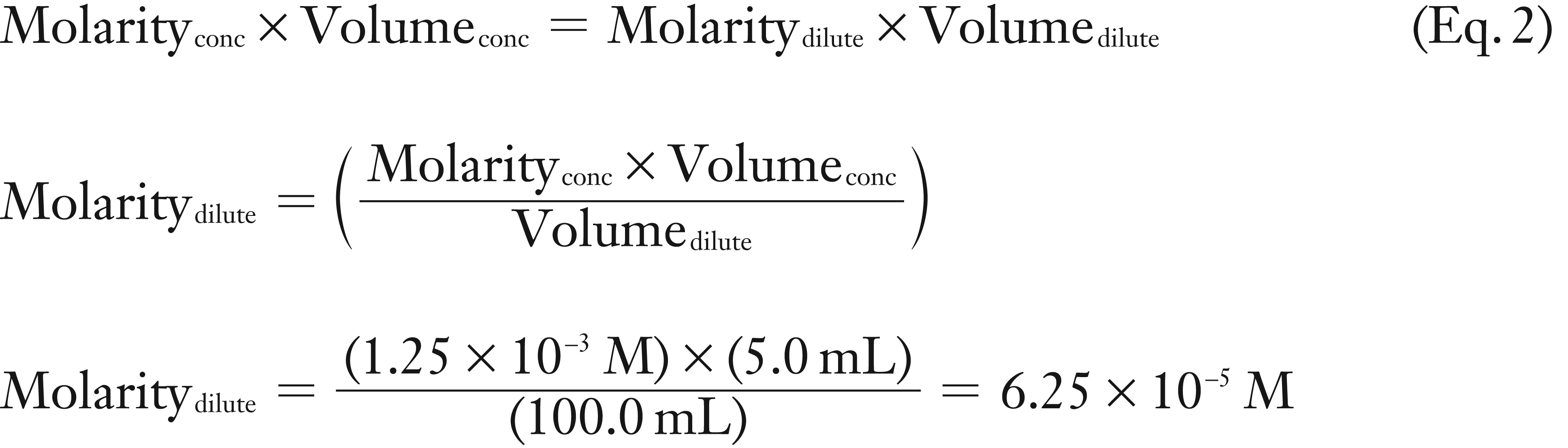
Problem
A calibration plot of absorbance vs. salicylate ion concentration was obtained. The slope of the best-fit straight line was 1200 M–1, resulting in the equation given below.
Absorbance = 1200 M–1 × concentration
An aspirin was dissolved in 5 mL of 1.0 M NaOH and then diluted to 100 mL to form a concentrated solution of salicylate ion. A 12.0 mL aliquot of the concentrated solution was diluted to 100 mL with FeCl3 solution to form the dilute, colored salicylate solution. The absorbance of the colored dilute solution was 0.58. What is the concentration of aspirin present in the original concentrated solution? How many mg of aspirin were present in the tablet?
Solution
(1) Using the equation of the line from the calibration plot and the absorbance of the colored dilute salicylate sample, you can solve for concentration of the dilute salicylate sample.
Absorbance = 1200 M–1 × concentration
0.58 = 1200 M–1 × concentration
concentration = (0.58) / (1200 M–1)
concentration = 4.83 × 10–4 M (keep an extra significant figure)
(2) Now you have to solve for the molarity of the original aspirin solution. You will rearrange Equation 2 to solve for the Molarity of the concentrated solution, knowing the Molarity of the dilute solution from the previous step. (The volume of the aspirin aliquot was 12.0 mL.)
(3) Finally, using the molarity of the concentrated solution, the volume of the concentrated solution (100 mL) and the molar mass of aspirin (180.16 g/mol), you will solve for mass of aspirin.
mg aspirin = Molarity × Volume × Molar Mass × (1000 mg/1 g)
mg aspirin = 4.02 3 10–3 M × 0.100 L × 180.16 g/mol × (1000 mg/1 g)
mg aspirin = 73 mg (rounded to 2 significant figures)
Procedure
Always Wear Safety Goggles and Use Good Lab Practices
Chemical Alert:
Part I
Sample Preparations
A. Preparing Concentrated Sodium Salicylate Solution
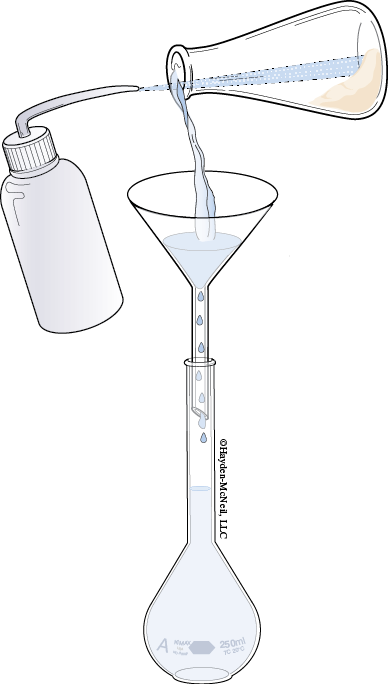
1. Fold a piece of weighing paper in half to make a crease. Tare the weight of the weighing paper. Add enough sodium salicylate to obtain a mass of approximately 0.100 g. Record the exact mass. Carefully transfer the solid into a clean 250 mL volumetric flask. Remove funnel.
2. Use a stream of deionized water from a wash bottle to rinse any solid clinging to the inside neck of the flask down into the solution.
3. Fill the flask about half full with deionized water. Swirl the flask until all of the solid dissolves.
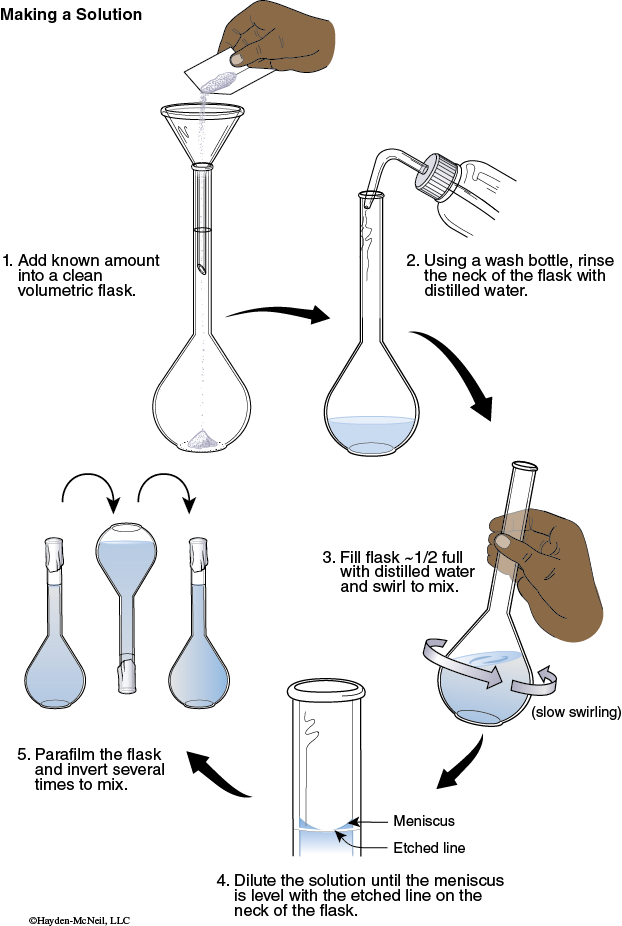
4. Add enough deionized water to fill the flask to the calibration mark. Add the last amounts dropwise. Seal the mouth of the flask with Parafilm.
5. Invert the flask several times to mix. Label the flask as concentrated sodium salicylate solution.
B. Preparing Concentrated Aspirin Solution
6. Obtain one aspirin tablet and place it in a 125 mL Erlenmeyer flask. Add 5 mL of 1.0 M NaOH to the flask. Use a stirring rod to break up the tablet and stir the solution. It will be cloudy white from some of the binding material in the tablet.
7. Heat the flask on the hot plate on medium heat for ~5 minutes. Use a towel to remove the hot flask from the hot plate and to cool down on the benchtop. Wash down the sides of the flask by directing a stream of distilled water from a wash bottle.
8. Pour the solution into a 100 mL volumetric flask using a funnel. Rinse out the Erlenmeyer flask with distilled water and pour the rinsing into the volumetric flask.
This is to ensure that all of the dissolved aspirin and solution are transferred to the volumetric flask.
Remove funnel. Add enough distilled water to fill the flask to the calibration mark.
9. Parafilm the top of the volumetric flask and invert several times to mix the solution. Label the flask as concentrated aspirin solution and set aside.
C. Preparing the Diluted Sodium Salicylate Solutions
10. Pour about 20 mL of the concentrated sodium salicylate solution into a 50 mL beaker. Rinse a plastic 5 mL graduated syringe by obtaining 5 mL of the solution in the syringe (pull back on the plunger) and expelling the rinsing into a 400 mL waste beaker (push plunger in). Leave the syringe in the beaker.
Pour ~140 mL of 0.020 M FeCl3 solution into a clean 250 mL beaker.
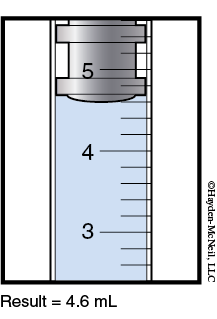
11. Transfer a 2.0 mL aliquot (portion) of the concentrated sodium salicylate solution (using the syringe) to a clean 25 mL volumetric flask. Dilute to the calibration mark with the FeCl3 solution.
Seal with Parafilm and invert several times to mix the solution. Calculate the molarity of this dilute solution and label the flask.
12. Next prepare an additional 3 solutions by repeating step 11 using 3.0 mL, 4.0 mL, and 5.0 mL aliquots of the concentrated sodium salicylate solution.
Calculate each molarity and label each flask with its concentration.
D. Preparing the Diluted Aspirin Solution
13. Pour about 20 mL of the concentrated aspirin solution into the 50 mL beaker. Rinse a different plastic 5 mL graduated syringe with this aspirin solution.
14. Transfer 1.0 mL of concentrated aspirin solution into a clean 25 mL volumetric flask using the syringe. Dilute to the mark with the FeCl3 solution.
Seal with Parafilm and invert several times to mix the solution. Label the flask as dilute aspirin solution.
Part II
Calibrating and Using the Spectrophotometer
15. Obtain a LabQuest and a SpectroVis Plus. Connect the SpectroVis Plus into the USB port of the LabQuest.
16. Obtain six spectrometer cuvettes. Each cuvette will be rinsed with the solution it will hold. To rinse, fill the cuvette about 2/3 full with its solution, and then pour out the solution into the waste beaker. Repeat a second time. The third time, fill the cuvette about 2/3 full and do not discard. Wipe off any spilled liquid or fingerprints with a Kimwipe. Only hold the cuvettes on the frosted or ridged sides.

17. Rinse and fill one cuvette with the FeCl3 solution—this is your “blank.”
Rinse and fill a cuvette with each of the four dilute sodium salicylate solutions.
Rinse and fill the last cuvette with the dilute aspirin solution.
18. Using the stylus, tap the MODE box on the LabQuest screen.
On the new MODE SCREEN, select Events with Entry from the pull-down menu at the top. In the NAME box, use the stylus to type Concentration and in the UNITS box, type M or mol/L (for molarity). See example below.
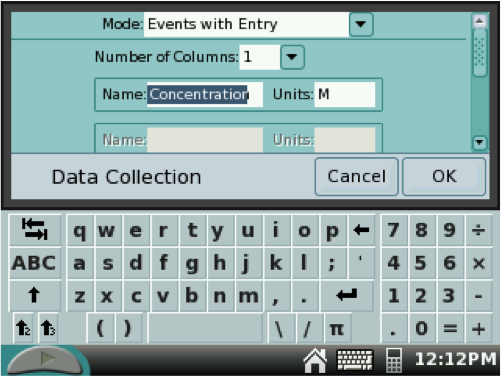
Scroll down and tap the check box for Average the collected data over 10 seconds.
Tap OK when finished to get back to the METER SCREEN.
Tap the screen in the ABSORBANCE box (red box) and select change wavelength from the menu. Enter 530 nm for the wavelength.
19. Insert the “blank” cuvette into the SpectroVis (ridges to the side).
Tap the screen in the ABSORBANCE box (red box) again, this time select Calibrate. When the CALIBRATION SCREEN appears, allow the lamp to warm up.
After the countdown is completed, tap the FINISH CALIBRATION button. Once the “Calibration Finished” message appears, tap OK.
Absorbance should read 0.00 in the ABSORBANCE box. Remove cuvette.
20. Place one of the dilute sodium salicylate solution cuvettes into the SpectroVis. Tap the PLAY button (green triangle). Tap KEEP (blue square next to the PLAY button).
A new screen will prompt you to enter the concentration using decimal format (0.00020). After entering the concentration, tap OK. You will be redirected to the GRAPH SCREEN.
Tap the STOP button (red square). Remove cuvette.
21. Place a second dilute sodium salicylate solute cuvette in the holder. Tap the PLAY button. A pop-up screen will ask you if you want to store, append, or discard data. Select Append.
This adds data instead of discarding previous measurements.
Continue by entering the concentration as in the previous step.
Repeat for the last two diluted samples. Remember to append each measurement. Each data point will be plotted.
Part III
Calibration Plot Using the LabQuest
22. After the last dilute solution is measured, you’ll see the graph of Absorbance vs. Concentration. Tap Analyze (at the top of the screen).
From the pull-down menu, select Curve Fit and then tap the check box for “Abs at 530 nm.”
On the CURVE FIT SCREEN, Select Linear from the pull-down CHOOSE FIT menu.
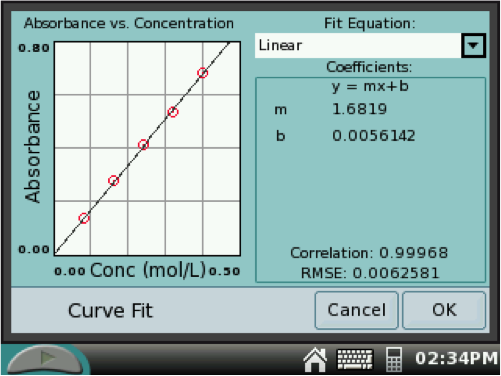
A line will appear on the graph along with the linear equation for the line (y = mx + b). Record the values of m and b.
The value of b, the y-intercept, should be close to zero. A measure of the linearity of your data is calculated in the Correlation. The more linear the data, the closer the value is to 1.000. Record your Correlation value.
Tap OK to return to the GRAPH SCREEN.
23. Repeat step 20 using the diluted aspirin cuvette. When prompted for the concentration, type Unknown. Record the absorbance of the aspirin.
24. The data for all measurements may be accessed by tapping the 3rd tab for the DATA SCREEN (data table icon).
Record ALL measurements and the values for your linear equation.
25. Pour all solutions into the appropriate waste container in the hood.
Return the LabQuest, SpectroVis and cuvettes.
Rinse and return the volumetric flasks and syringes. Clean and return all other glassware to your drawers.
Part IV
Manually Plotting Calibration Graph
26. Remove a piece of graph paper from the back of your lab manual.
Plot absorbance (y-axis) versus concentration (x-axis) for the dilute sodium salicylate solutions. The graph should fill the graph paper.
27. Draw a best-fit straight line through your data points. The line MUST go through the origin (0,0) of the graph (when there was no sodium salicylate, the “blank,” there was no absorbance). Note that not all of the points may fall on the line.
Determine the slope of the line and compare it to the value from the LabQuest.
28. Rewrite Equation 1, inserting the numerical value of the slope as the constant.
Using new Equation 1, determine the concentration of the dilute aspirin solution using its absorbance.
29. Calculate the concentration in the concentrated aspirin solution (Mconc).
30. Calculate the mg of aspirin in the tablet used to prepare the concentrated aspirin solution. Was your tablet extra strength, regular strength, or low dosage?
Discussion
1. Summarize the results of this experiment.
2. What is the quality of the results? Do the results seem reasonable?
3. What were the errors or possible errors in the experiment?
4. How would the errors affect the results of the experiment?
Study Questions
1. a. A 0.40 g sample of sodium salicylate was dissolved and diluted to a final volume of 250 mL with deionized water. What is the concentration of this solution?
b. An 8.0 mL aliquot of the sodium salicylate solution above was diluted to a final volume of 100 mL with FeCl3 solution. What is the concentration of this dilute sodium salicylate solution?
2. A calibration plot of absorbance vs. concentration was obtained, with the slope of the best-fit straight line as 1220 M–1. The absorbance of a dilute aspirin solution mixed with FeCl3 was 0.72. What is the concentration of the dilute aspirin solution?
3. a. A 5.0 mL aliquot of concentrated aspirin Brand X solution was diluted to a final volume of 100 mL with FeCl3. This solution’s concentration was determined to be 5.5 x 10–5 M. What is the concentration of the aspirin in the original aspirin Brand X solution?
b. The original (concentrated) aspirin Brand X solution was made by dissolving one tablet of Brand X aspirin in 10 mL of 1.0 M NaOH, boiling and then diluting the solution to a final volume of 100 mL. How many mg of aspirin are present in a tablet of Brand X?
4. One way to judge the quality of the results is to look at the calibration plot. The closer all the points are to the line, then the “better” the plots and results. A plot with a straight line with very few points on the line or close to the line has a lot of uncertainty in it. Looking at your plot and your results, how confident are you of your results? Explain your level of confidence in your plot and results.
5. One tablet of Brand Y aspirin was dissolved in 10 mL of 1.0 M NaOH, boiled, and then diluted to a final volume of 100 mL with deionized water. A 2.5 mL aliquot of this concentrated aspirin solution was diluted to a final volume of 100 mL with FeCl3. A calibration plot of absorbance vs. concentration was obtained, with the slope of the best-fit straight line as 1370 M–1. The diluted aspirin solution had an absorbance of 0.932. What is the concentration of the dilute aspirin?
a. What is the concentration of the aspirin in the dilute aspirin solution?
b. What is the concentration of the original aspirin solution?
c. How many mg of aspirin are present in one tablet of Brand Y aspirin?
6. The absorbance of a dilute aspirin solution (diluted with FeCl3) generates an error—too concentrated. After showing your TA that you cannot determine the absorbance of the solution, the TA tells you to make a new solution dilute aspirin solution. When making a new solution, should it be more concentrated or less concentrated?
Activity Completed!