Chapter
5. Basic Properties of Light
5.1 Introduction
AstroTutorials
true
To advance to the next page of the tutorial you need to submit every question; currently you have not finished all the questions on this page. Leaving a tutorial page without submitting all the questions results in you receiving no grade in the gradebook.
true
Author: Grace L. Deming, University Of Maryland
Editor: Beth Hufnagel, Anne Arundel Community College
The goals of this module: After completing this exercise, you should be able to:
- Describe the different regions of the electromagnetic spectrum (light).
- Compare the wavelength, frequency, and energy differences among the regions of the electromagnetic spectrum.
In this module you will explore:
- The different regions of the electromagnetic spectrum.
- How light is described using wavelength, frequency, and energy.
Why you are doing it: An understanding of the phenomenon that scientists call electromagnetic radiation (light) is crucial in gaining knowledge about our universe. Astronomers use the complete electromagnetic spectrum to investigate the nature of celestial objects.
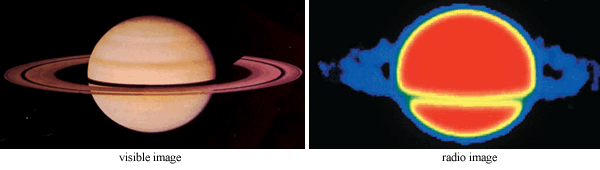
Two Views of Saturn
5.2 Background

William Herschel studies a spectrum. Copyright SPL/PHOTO RESEARCHERS, INC.
Early astronomers like Galileo, Newton, and Herschel were curious about light. Around 1670, Isaac Newton passed sunlight through a prism and displayed a rainbow of colors. After William Herschel discovered Uranus in 1781, he added to Newton's experiment and investigated additional properties of light. Look carefully at the image to the right that shows Herschel using a prism to create a spectrum. Can you spot the prism in the small window over Herschel's left shoulder? By directing sunlight through the glass prism, Herschel created a rainbow of colors. The order of the colors is always the same.
Question
5.1
KMjh7+TmkcLseTcl0hBwrFefQEr2LQvsgI0W40bcqljFPD21GGXlY5VCK6os8HuHXsiWLnJRdiWe1P070ophrhXN6oNtVevvl8eFCKGbutf2a4ZHyo55M9DkcEo1kSJweedAwmIvdH9RmgBCzhNQbNHXw47IxTUOggxj8lhekahMPCQrSkTEKJwJAmRWabQlA6jYWiiYcR+2hQLmQKb5ONrz8ZfXpq9sODcHfdJ3GW6K9gdbDaisLZiRyedMQ2GlFEp+8cfw9ebHYEKPkAHvHiBS7+OGQjX+sXRsIYcpHhjrgCDfRIR3f5xWA4MacWRl1dqCPsySKII=
3
No. Look carefully at the side of the box to make sure that you are including all colors. Try again.
Correct. You can see orange too, if you look carefully on the side of the little platform between the red and yellow. If you are color-blind and can't see all of the colors, no problem! As you will see, the colors are due to tiny differences in the light's wavelength, which has a numerical value.
Incorrect. You can see orange too, if you look carefully on the side of the little platform between the red and yellow. If you are color-blind and can't see all of the colors, no problem! As you will see, the colors are due to tiny differences in the light's wavelength, which has a numerical value.
Summary
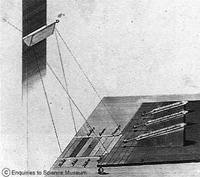
Herschel's original prism and thermometers
On the little platform you will notice three thermometers. In 1800, Herschel was able to show that when a thermometer was placed under each color, the temperature was higher than for a thermometer not in the spectrum at all, which serves as a 'control' in this experiment. The placement of the thermometer in the image just beyond the red color also showed a higher temperature than for the control thermometer. Herschel had discovered a new, invisible form of light, infrared radiation!
5.3 Prism
You may have noticed in your home that as sunlight passes through a glass vase, a rainbow is produced on the other side of the room. A beam of sunlight is called "white light" and contains all of the colors of the rainbow. The glass breaks up the sunlight into its component parts and you see the color bands of the rainbow. This rainbow is called the visible spectrum.

A prism produces a spectrum
Look at the glass prism above. A spectrum is produced as the white light shines through the prism. The scale on the right gives the wavelength of the color displayed. The units are in nanometers. One nanometer is equal to a length of 10-9 meters, one billionth of a meter.
Question
5.2
vE0ZbOD1/EXtj1FT19yctChMXaE/K9Mcdfyjzz1RHc2VEQUdG3ggOWlDcblTOymqrodjY0BdlwlJ/X8DpfyxWwnV2anzLtfNTAW04Cxtj9dEGdCJdflpOrAYHNcJKdP6CmZbWxrPPBbX3ekb70SNZvJOsJwYsohAjbXnKvJ8ZnB6hiKO/1cEN5BUDLoVjQlMtKE3YxlWfSnawwSwjjU6HnsHLcvRDo46
3
No, look at the scale carefully and try again.
Correct. At 700 nm, the wavelength for red light is greatest for visible light. At 400 nm, violet has the shortest wavelength.
Incorrect. At 700 nm, the wavelength for red light is greatest for visible light. At 400 nm, violet has the shortest wavelength.
Summary
Light can be described in terms of wavelength, the distance between two successive wave crests. The Greek letter lambda λ , is used to symbolize wavelength.
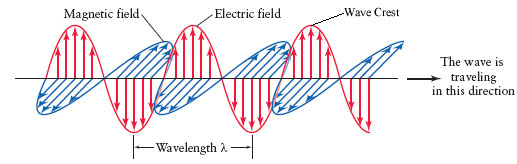
An electromagnetic wave
5.4 Wavelength and Frequency
Frequency refers to the number of wave crests that pass a point every second and is inversely related to wavelength by the equation, ν = c/λ , where ν = frequency, λ = wavelength, and c = speed of light. The animation below shows two waves of differing wavelengths. There is a "wave crest counter" toward the right side of the animation that will record the number of wavecrests that pass by.

Question
5.3
omR+50stA9GTnqM/XInIYSgyM0rjw+PEky/qyKh4Rs/Ntut02y6JyFP1xDoFAEcyPGvMYHvA+Z5S8aMvPHX/rjsFIYbLryrkmF5cAvc+zk724g1sqLLNvKZQ66Q9IlwqsJPpLnREe4pa6Kj5AbWGIY12HLYErutyq2svDGOCYbD+JXyPSsNZHFVTmJEBn2gMYJphcAgMqoQesKNnGoTKqw==
3
No. Play the animation again and notice that, as the wavelength increases, the frequency decreases. This is what is meant by an inverse relationship. Try again.
Correct. Now think about the equation, ν = c/λ. Since c is a constant, the speed of light, ν depends on 1/λ. As one goes up (increases), the other must go down (decreases).
Incorrect. Now think about the equation, ν = c/λ. Since c is a constant, the speed of light, ν depends on 1/λ. As one goes up (increases), the other must go down (decreases).
5.5 Wave Energy
The energy associated with light of a specific wavelength is given by the equation, E = hc/λ, where E = energy, h = Planck's constant (6.625 × 10-34 Joule-seconds), and λ = wavelength.
Investigate the relationship between frequency, wavelength, and energy by sliding the bar over the colors in figure above.
Question
5.4
zu/n83MnAf/uiRkQhT1IfpVd7MCaiJ7qRC8d+APoLi1KDAEv0HqJs5R0z+3wAIwTqv0dHl70UzYEBgtp2nZS7qmET7hH6JbM+syZkuhkjwm/LzhXtB1COBkNI5xV9wx463G9ja9e9kJVWgY89XLDBg==
Correct. Since E = hc/λ, and E and c are constants; E depends on 1/λ. When one goes up, the other must go down.
Incorrect. Since E = hc/λ, and E and c are constants; E depends on 1/λ. When one goes up, the other must go down.
5.6 Electromagnetic Spectrum
Here is the entire electromagnetic spectrum. All of the named regions are forms of electromagnetic radiation and all forms travel at the speed of light through empty space.

Question Sequence
Question
5.5
3p0kBMeBPsH1Dm/DCDqKW3A60kJoPVEvRZbRRt7S6erFc2ZBgvI+qtCk2rrPol1MiZ20mOTsFhMwz9sYTizUJryFkQdi91auIQ6KOR6mCKS3FDs0I6mmuFYmE5+5BLNHqXpVVfVBwNNEcK2+az1Fsh/5dZTHEbk6pUcv4FOVel2iloCdY78Zuyz8BJtF5dXzTak9QcG58Ii8RBWYZ4vzlHyKX9KzYP+8
Correct. Isn't it surprising how little of the electromagnetic spectrum we can detect with our eyes?
Incorrect. Isn't it surprising how little of the electromagnetic spectrum we can detect with our eyes?
Question
5.6
Modern technology uses the full range of the electromagnetic spectrum, from radio waves to Gamma rays. Now try 'clicking' on the X-ray portion of the spectrum. Explore other applications by 'clicking' on the other nonvisible regions of the spectrum.
LVakAZlC2iilG+ynxzVr4q2mnbVUjeqF09rgbqJoZTtP6F2+c82nVKlZqUTAlOlJ42E+zTsrFoFrETHoeyPheUGMW/RmXYGM+DYzzqQ/uIu6zdwO5pQdJMCOJWKTsGxmkRML2Z0noOIUqesLfYQBKtmBEFocP2k48TbtrQsbMvRa8BniNi7lGKlwzrf5sS+9WTYc4haFVt3S7aY13n6pg4rueYnntFJsoZHqeV2BlGfeXL4+de0zFpju3mo/iJAkYYJ/1WrqpL8lwkmJPv+++nY0BGiW+5gI/cUvMDj+3ZRaZmJOD0vG2zVLnnze7cj+T81t7yaVZ4nJet4YBYgtbYu1BVmFxw3p
3
Try again.
Correct. Gamma rays have the highest energy of any region in the electromagnetic spectrum and the absorption of this energy damages the cancer cell's DNA, preventing replication.
Incorrect. Gamma rays have the highest energy of any region in the electromagnetic spectrum and the absorption of this energy damages the cancer cell's DNA, preventing replication.
5.7 Quick Check Quiz
Indepth Activity: Basic Properties of Light
Question
5.7
ccvvV6LOsuCv6gOgUZmn3OksPg+FWgF8nJE5jeR7+F8fWM40b42H02wfmEBjNYq2xCIz8YgybmzgrqeRFa+OwaXX+iAv9dK/dnApKS6W6Gp3fAhRSbEkHHvQhFwukIE/oerxbhNbRXHoB5yUzk8qHRFXFFXENuCmeMGRiBp4LudKFNP4a0MTESoRUZwuf6Rdu4ayVUl4AfU6fju283yguc/wy/cHLka9nGdu9tYzxNGEliEbATtuov22YxJr4vayQXCTSr1nesdD2SPZhs2Mz+FAEAlVwaTySLAdnQvT7ltnazZnN+NjC93H40Thexlsk/JtjivRoMd7QaE/iQGZr73gjXHKaBInzADLC7xR/3PM8Iij
Correct. Visible light is actually a very small portion of the electromagnetic spectrum.
Incorrect. Visible light is actually a very small portion of the electromagnetic spectrum.
Question
5.8
ttvtBW6vxluCgj785Ill4tNRgdARtQ7Kwkr6cUDz1LmUc8qFzTVugdXvM0q9s6p1ou/36O3SfIgvwdYLtWFOIu8kti2SAjXtA5MRT74c2RJCW7mWibnx+NK9+9Q9bS0K55K7xylpVo9gB1haPT9hQ5NmvN7bCvvTIoM6c1MDzIfMbG27+K0K+1SpkFF00R3mirR9IgLE4dI1F7/IkcMv0+Cs1iJmMySN1nGidqRlluAcG4I7r28aHepinLGqk6Zvsd+x3s9k6u9CMg1Y8lvA9tN/jMGys7fnyVikKpqBsal5SURXxTN8hd3V1t/F6yfj19Eg9Je6Ew3i6+KonLxNm3dRfHBttKdgb1UfshBvB1fdVfFBshKBCeRcYN1NE4Ds2PGDxjSlsv1WsDeDQgW1rhF9UQeaqk9+rxEY8diRouIGh7fonWPnxIG8X8c=
Correct. Gamma rays have the greatest energy, shortest wavelength, and highest frequency of any form of electromagnetic radiation.
Incorrect. Gamma rays have the greatest energy, shortest wavelength, and highest frequency of any form of electromagnetic radiation.
Question
5.9
o1TRwSewcN3tcyH7/faQdAbrknEbQG9x9ZA7B25JZa7f1lT1AFnIE2GG0xF/7PeJcbIM9uqm0Zzw4z9FD5r6F82BKc9CoWY3OQVjL6v/6tvoWJvYd7QKXnkKXNghfPLUhkQkiI2F6sM/w9USDZoveK+Hnk6YkGaNND8tg6+h/lSWAOW8VAIZLeXa+8GMPfY7r/OE5Q==
Correct. Radio waves are the longest wavelength and have the largest range of wavelengths, from centimeters to kilometers in length.
Incorrect. Radio waves are the longest wavelength and have the largest range of wavelengths, from centimeters to kilometers in length.
Question
5.10
NULjj+AXjsHrfb7Qya/AQp25C0ofeExt1JfJMIgsC8krWhAoSQsYOwySZbIhkcGKttgSCqEcrrbix4Nsd9CiVNbl4GSVEs9CH2T8odjdZIzomiKQZ2pGdheypl+JPEYLhxgP+m8CYx3Ta/h8GojilmitHAMIdawTj75vwjH/+aNtrL0khIn7gT3KSYeRkSP23s34m7OS+Tm/d/cOpiLAUBI1afPMckFE729TyrbKZovBQh78FGGw82/LDoU4/fCNIqz+jvnQNj7l07/5uTTfDT4wf/yDmgxLyoUuc8hUIMgdBD27TbwLh+fn89SrkmjN
Correct. Since E = hc/λ, and E and c are constants; E depends on 1/λ. When one goes down, the other must go up.
Incorrect. Since E = hc/λ, and E and c are constants; E depends on 1/λ. When one goes down, the other must go up.
Question
5.11
KJEdSPb/6lyuIpZB7kYj5KiGKAayv1nOFo0uAh3UGrSfQGInSCZyYUzogoTmzE1VQWOInHZEdpR0cHmIU8xHFFkt6mSV2zWbomENin8y/QQJbSomNyApIEXDyCy5fYtsPlp7UkbYKXHt+jItRuzKWjFI/1Y2LhWJ+k1A3GFInj5Jv2nPXdiEaBzb2Ie9Rym71f6EBOSylXHSeq37lGwbNdvBs6WFpSEvy2yuvFHAUzTbbWbOq4OOnx0YwUNyIxbMBYb1+p/FjLqR4pfkhcxhV0C/26ZhahFPlAa2sb8PCO15+6lElH2muTMAYzd/ncUr
Correct. Recall the equation, ν = c/λ. Since the speed of light, c, is a constant, ν depends on 1/λ. As one goes up (increases), the other must go down (decreases).
Incorrect. Recall the equation, ν = c/λ. Since the speed of light, c, is a constant, ν depends on 1/λ. As one goes up (increases), the other must go down (decreases).
Question
5.12
0JFMx1n6e6VV3RzZAfrVqiC+NPlxqwh3IFAnhij0lbCXodjasSjGBSB7aZ9BYw6qA2nUkNECvKONoIQfeD1JvhUHQ/cbD7Wh490dP1hG6DPH3TrQyOZCoGpMjwszEeCyzUMhJvhd3bkATjSnlFDmxl0cGFuNF7Yxbxt2iZcXzFV0KTX88xMstN/3xUHjRoQF81A14exOz23gNlXO0pFwrJvQ3SeHNp/Dm7qTQF7Rd+LRRWU61zOpGICKE3CQ3BDKXkTT3K0nhUnuXGfIzGgeYg==
Correct. Infrared remote controls work well enough to have stuck around for 25 years, but they do have some limitations related to the nature of infrared light. Infrared remotes have a range of only about 30 feet (10 meters), and they require line-of-sight.
Incorrect. Infrared remote controls work well enough to have stuck around for 25 years, but they do have some limitations related to the nature of infrared light. Infrared remotes have a range of only about 30 feet (10 meters), and they require line-of-sight.
Question
5.13
zBkidDWncCP4aBVAUGqEpkt9xdp0o753/AFtSR+HPKiX+TIMYzGZ1xpy9TIb+ik7ANqzk40Dyk+QGHwP8GzGWuAUdSx81ziUBMSpzcGHmtlmoksUXht0i6ggekSy67u2DcwFVlXvUQ7qA9GlT9FeKAlR6YrPXHo8HLCwhEQqq3n0pC3zFq+ciTELRL+0unJA8gP4b1yyfvQDw65gs1OZdaofu/VCRniXscnr8w0Y5LM=
Correct. Even though there are many uses for the different regions of the electromagnetic spectrum, the only region humans can detect is visible light. This means that every “image” created using the other wavelengths must be rendered into an image that is still viewable in visible light for the human eye.
Incorrect. Even though there are many uses for the different regions of the electromagnetic spectrum, the only region humans can detect is visible light. This means that every “image” created using the other wavelengths must be rendered into an image that is still viewable in visible light for the human eye.
Question
5.14
hj8eRInK41lrRbGKpbZnmDnXxJwGzL+oR2YMOvvh4y5I2383a41rpjFbM2udpOYfTN4gsZRn6gZfKiNPU9DmsiJXWo7906kBsn6zQZoRgp6MnLcVbQ+geNppFDo6npKiOXjRwCnqH4cF8/y1SPCG04frXHrMVdQn+nN9GkBPtdJgCSJnRkmkQcAxNXHkSDZY9g+pU3ncsTANkkCLVFzgYoCkC0OxoY6Tm7qspsBbiI/iYd7trr21EDLdcRpzMoSvx0dVjZBH5KDcW/wKhDc47UfgqXogS0mZO/whecpJpyeMlstFMCAnMcvRdm2l+aOfOoG/j3ncV7g=
Correct. Wavelength and frequency are inversely proportional - when one increases, the other decreases. Wavelength and energy are also inversely proportional - when one goes up (increases), the other goes down (decreases).
Incorrect. Wavelength and frequency are inversely proportional - when one increases, the other decreases. Wavelength and energy are also inversely proportional - when one goes up (increases), the other goes down (decreases).
Question
5.15
Y/s4bSNFSHJt8b3esOa1qjGT3a12c0HgJRIQySvkriAypYHBZUbZcSt1mlioG58umJ0ih5L406Sqxnr7MijtZduf7P3Kac1sS1tO2W3o/iZl4bU20sACDVr49z4hLLX8jbg+rqGOmgCEgPnkF7sWWZIOqtZ1PG6bB6fO+ItPgt+ugH4o3ME0mbWTQtkTx8GIxMUszj4Yh53uCIG0
Correct. The invisible region of the spectrum closest to red light is infrared - slightly longer wavelength than red visible light.
Incorrect. The invisible region of the spectrum closest to red light is infrared - slightly longer wavelength than red visible light.
Question
5.16
BM3TIUtuE6fO8rQ7gD6CNbm4/E8IplZw87B5k9F8RL+QPMdBG1yhZVXEi2DLp5l+TNQdo4dYlxWDNoda3DAe3ysahJHc7FF5sfxtMequWemthbamGzcKw9vtDX/zkToqpOByjKCZRu0cxuqXtIvEBdc833lh2/YZdEVT4Ejj7voyCYDsoGy3L1qzObvxg8owrUavvA==
Correct. An example would be the relationship between wavelength and energy.
Incorrect. An example would be the relationship between wavelength and energy.






