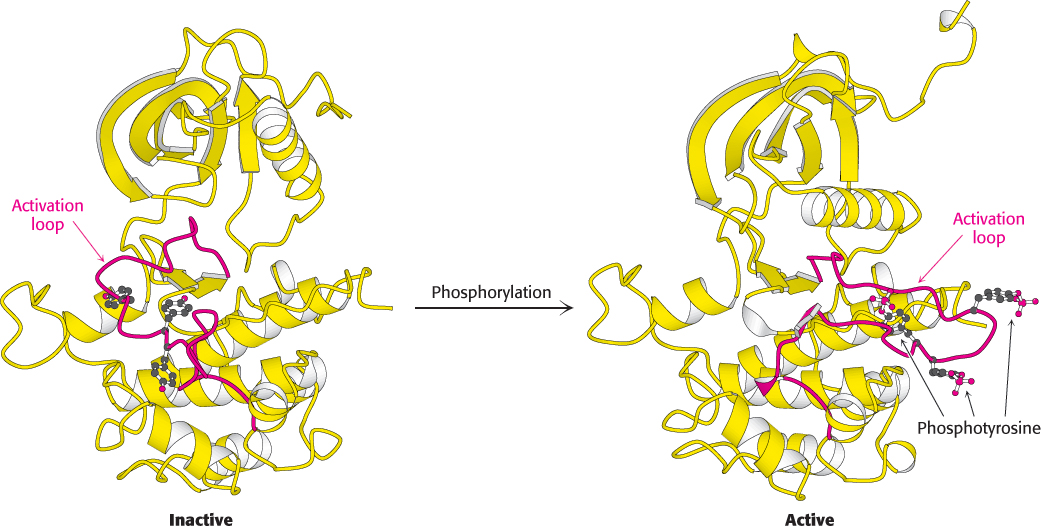
Activation of the insulin receptor by phosphorylation. The activation loop is shown in red in this model of the protein kinase domain of the β subunit of the insulin receptor. The unphosphorylated structure on the left is not catalytically active. Notice that, when three tyrosine residues in the activation loop are phosphorylated, the activation loop swings across the structure and the kinase structure adopts a more compact conformation. This conformation is catalytically active.
[Drawn from 1IRK.pdb and 1IR3.pdb.]

