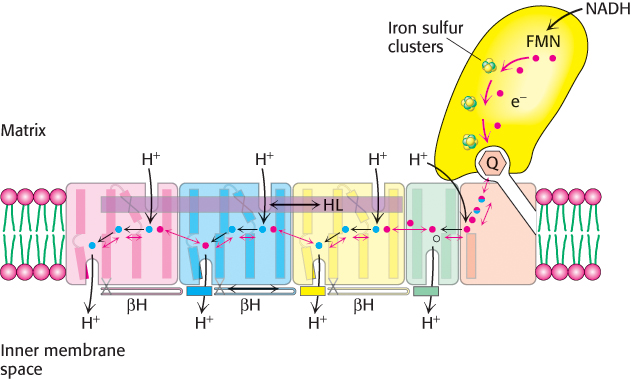
Coupled electron– H- n– 2- 2-
[Information from R. Baradaran et al., Nature 494:443–