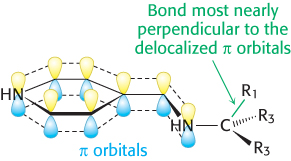
Stereoelectronic effects. The orientation about the N—