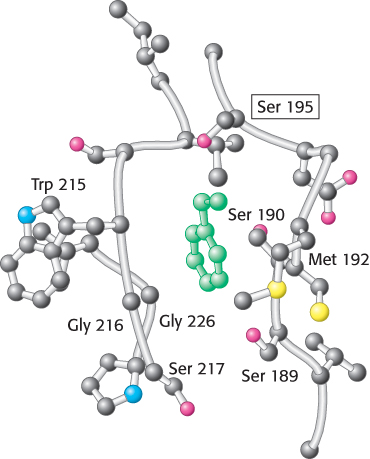
Specificity pocket of chymotrypsin. Notice that this pocket is lined with hydrophobic residues and is deep, favoring the binding of residues with long hydrophobic side chains such as phenylalanine (shown in green). The active-