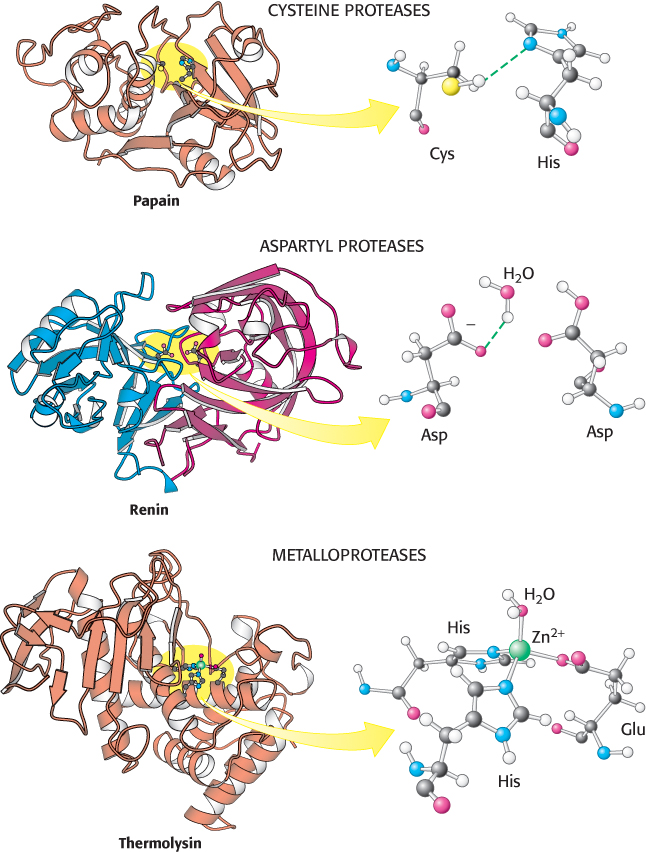
Three classes of proteases and their active sites. These examples of a cysteine protease, an aspartyl protease, and a metalloprotease use a histidine-activated cysteine residue, an aspartate-activated water molecule, and a metal-activated water molecule, respectively, as the nucleophile. The two halves of renin are in blue and red to highlight the approximate twofold symmetry of aspartyl proteases. Notice how different these active sites are despite the similarity in the reactions they catalyze.
[Drawn from 1PPN.pdb.; 1HRN. pdb; 1LND.pdb.]

