PROBLEMS
Question 1.1
Donors and acceptors. Identify the hydrogen-
Question 1.2
Resonance structures. The structure of an amino acid, tyrosine, is shown here. Draw an alternative resonance structure.
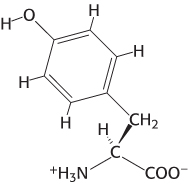
Question 1.3
It takes all types. What types of noncovalent bonds hold together the following solids?
Table salt (NaCl), which contains Na+ and Cl− ions.
Graphite (C), which consists of sheets of covalently bonded carbon atoms.
Question 1.4
Don’t break the law. Given the following values for the changes in enthalpy (ΔH) and entropy (ΔS), which of the following processes can take place at 298 K without violating the Second Law of Thermodynamics?
ΔH = −84 kJ mol−1 (−20 kcal mol−1),
ΔS = +125 J mol−1 K−1 (+30 cal mol−1 K−1)
24
ΔH = −84 kJ mol−1 (−20 kcal mol−1),
ΔS = −125 J mol−1 K−1 (−30 cal mol−1 K−1)
ΔH = +84 kJ mol−1 (+20 kcal mol−1),
ΔS = +125 J mol−1 K−1 (+30 cal mol−1 K−1)
ΔH = +84 kJ mol−1 (+20 kcal mol−1),
ΔS = −125 J mol−1 K−1 (−30 cal mol−1 K−1)
Question 1.5
Double-
Question 1.6
Find the pH. What are the pH values for the following solutions?
0.1 M HCl
0.1 M NaOH
0.05 M HCl
0.05 M NaOH
Question 1.7
A weak acid. What is the pH of a 0.1 M solution of acetic acid (pKa = 4.75)?
(Hint: Let x be the concentration of H+ ions released from acetic acid when it dissociates. The solutions to a quadratic equation of the form ax2 + bx + c = 0 are 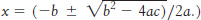
Question 1.8
Substituent effects. What is the pH of a 0.1 M solution of chloroacetic acid (ClCH2 COOH, pKa = 2.86)?
Question 1.9
Water in water. Given a density of 1 g/ml and a molecular weight of 18 g/mol, calculate the concentration of water in water.
Question 1.10
Basic fact. What is the pH of a 0.1 M solution of ethylamine, given that the pKa of ethylammonium ion (CH3 CH2 NH3+) is 10.70?
Question 1.11
Comparison. A solution is prepared by adding 0.01 M acetic acid and 0.01 M ethylamine to water and adjusting the pH to 7.4. What is the ratio of acetate to acetic acid? What is the ratio of ethylamine to ethylammonium ion?
Question 1.12
Concentrate. Acetic acid is added to water until the pH value reaches 4.0. What is the total concentration of the added acetic acid?
Question 1.13
Dilution. 100 mL of a solution of hydrochloric acid with pH 5.0 is diluted to 1 L. What is the pH of the diluted solution?
Question 1.14
Buffer dilution. 100 mL of a 0.1 mM buffer solution made from acetic acid and sodium acetate with pH 5.0 is diluted to 1 L. What is the pH of the diluted solution?
Question 1.15
Find the pKa. For an acid HA, the concentrations of HA and A− are 0.075 and 0.025, respectively, at pH 6.0. What is the pKa value for HA?
Question 1.16
pH indicator. A dye that is an acid and that appears as different colors in its protonated and deprotonated forms can be used as a pH indicator. Suppose that you have a 0.001 M solution of a dye with a pKa of 7.2. From the color, the concentration of the protonated form is found to be 0.0002 M. Assume that the remainder of the dye is in the deprotonated form. What is the pH of the solution?
Question 1.17
What’s the ratio? An acid with a pKa of 8.0 is present in a solution with a pH of 6.0. What is the ratio of the protonated to the deprotonated form of the acid?
Question 1.18
Phosphate buffer. What is the ratio of the concentrations of 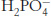 and
and 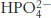 at (a) pH 7.0; (b) pH 7.5; (c) pH 8.0?
at (a) pH 7.0; (b) pH 7.5; (c) pH 8.0?
Question 1.19
Neutralization of phosphate. Given that phosphoric acid (H3PO4) can give up three protons with different pKa values, sketch a plot of pH as a function of added drops of sodium hydroxide solution, starting with a solution of phosphoric acid at pH 1.0.
Question 1.20
Buffer capacity. Two solutions of sodium acetate are prepared, one with a concentration of 0.1 M and the other with a concentration of 0.01 M. Calculate the pH values when the following concentrations of HCl have been added to each of these solutions: 0.0025 M, 0.005 M, 0.01 M, and 0.05 M.
Question 1.21
Buffer preparation. You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acid plus acetate concentration of 250 mM and a pH of 5.0. What concentrations of acetic acid and sodium acetate should you use? Assuming you wish to make 2 liters of this buffer, how many moles of acetic acid and sodium acetate will you need? How many grams of each will you need (molecular weights: acetic acid 60.05 g mol−1, sodium acetate, 82.03 g mol−1)?
Question 1.22
An alternative approach. When you go to prepare the buffer described in Problem 21, you discover that your laboratory is out of sodium acetate, but you do have sodium hydroxide. How much (in moles and grams) acetic acid and sodium hydroxide do you need to make the buffer?
Question 1.23
Another alternative. Your friend from another laboratory was out of acetic acid, so tries to prepare the buffer in Problem 21 by dissolving 41.02 g of sodium acetate in water, carefully adding 180.0 ml of 1 M HCl, and adding more water to reach a total volume of 2 liters. What is the total concentration of acetate plus acetic acid in the solution? Will this solution have pH 5.0? Will it be identical with the desired buffer? If not, how will it differ?
25
Question 1.24
Blood substitute. As noted in this chapter, blood contains a total concentration of phosphate of approximately 1 mM and typically has a pH of 7.4. You wish to make 100 liters of phosphate buffer with a pH of 7.4 from NaH2PO4 (molecular weight, 119.98 g mol−1) and Na2HPO4 (molecular weight, 141.96 g mol−1). How much of each (in grams) do you need?
Question 1.25
A potential problem. You wish to make a buffer with pH 7.0. You combine 0.060 grams of acetic acid and 14.59 grams of sodium acetate and add water to yield a total volume of 1 liter. What is the pH? Will this be the useful pH 7.0 buffer you seek?
Question 1.26
Charge! Suppose two phosphate groups in DNA (each with a charge of −1) are separated by 12 Å. What is the energy of the ionic interaction between these two phosphates assuming a dielectric constant of 80? Repeat the calculation assuming a dielectric constant of 2.
Question 1.27
Vive la différence. On average, estimate how many base differences there are between two human beings.
Question 1.28
Epigenomics. The human body contains many distinct cell types yet almost all human cells contain the same genome with 21,000 genes. The distinct cell types are primarily due to differences in gene expression. Assume that one set of 1000 genes is expressed in all cell types and that the remaining 20,000 genes can be divided into sets of 1000 genes that are either all expressed or all not expressed in a given cell type. How many different cell types are possible if each cell type expresses 10 sets of these genes? Note that the number of combinations of n objects into m sets is given by n!/(m!(n-
Question 1.29
Predispositions in populations. Assume that 10% of the members of a population will get a particular disease over the course of their lifetime. Genomic studies reveal that 5% of the population have sequences in their genomes such that their probability of getting the disease over the course of their lifetimes is 50%. What is the average lifetime risk of this disease for the remaining 95% of the population without these sequences?
26