2.3 Secondary Structure: Polypeptide Chains Can Fold into Regular Structures Such As the Alpha Helix, the Beta Sheet, and Turns and Loops
Can a polypeptide chain fold into a regularly repeating structure? In 1951, Linus Pauling and Robert Corey proposed two periodic structures called the α helix (alpha helix) and the β pleated sheet (beta pleated sheet). Subsequently, other structures such as the β turn and omega (Ω) loop were identified. Although not periodic, these common turn or loop structures are well defined and contribute with α helices and β sheets to form the final protein structure. Alpha helices, β strands, and turns are formed by a regular pattern of hydrogen bonds between the peptide N H and C
H and C O groups of amino acids that are near one another in the linear sequence. Such folded segments are called secondary structure.
O groups of amino acids that are near one another in the linear sequence. Such folded segments are called secondary structure.
The alpha helix is a coiled structure stabilized by intrachain hydrogen bonds
Screw sense
Describes the direction in which a helical structure rotates with respect to its axis. If, viewed down the axis of a helix, the chain turns in a clockwise direction, it has a right-handed screw sense. If the turning is counterclockwise, the screw sense is left-handed.
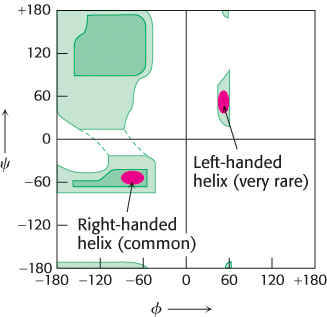
Figure 2.26: Ramachandran plot for helices. Both right- and left-handed helices lie in regions of allowed conformations in the Ramachandran plot. However, essentially all α helices in proteins are right-handed.
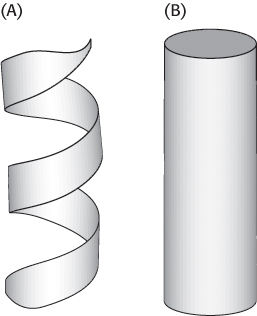
Figure 2.27: Schematic views of α helices. (A) A ribbon depiction. (B) A cylindrical depiction.
In evaluating potential structures, Pauling and Corey considered which conformations of peptides were sterically allowed and which most fully exploited the hydrogen-bonding capacity of the backbone NH and CO groups. The first of their proposed structures, the α helix, is a rodlike structure (Figure 2.24). A tightly coiled backbone forms the inner part of the rod and the side chains extend outward in a helical array. The α helix is stabilized by hydrogen bonds between the NH and CO groups of the main chain. In particular, the CO group of each amino acid forms a hydrogen bond with the NH group of the amino acid that is situated four residues ahead in the sequence (Figure 2.25). Thus, except for amino acids near the ends of an α helix, all the main-chain CO and NH groups are hydrogen bonded. Each residue is related to the next one by a rise, also called translation, of 1.5 Å along the helix axis and a rotation of 100 degrees, which gives 3.6 amino acid residues per turn of helix. Thus, amino acids spaced three and four apart in the sequence are spatially quite close to one another in an α helix. In contrast, amino acids spaced two apart in the sequence are situated on opposite sides of the helix and so are unlikely to make contact. The pitch of the α helix is the length of one complete turn along the helix axis and is equal to the product of the rise (1.5 Å) and the number of residues per turn (3.6), or 5.4 Å. The screw sense of an α helix can be right-handed (clockwise) or left-handed (counterclockwise). The Ramachandran plot reveals that both the right-handed and the left-handed helices are among allowed conformations (Figure 2.26). However, right-handed helices are energetically more favorable because there is less steric clash between the side chains and the backbone. Essentially all α helices found in proteins are right-handed. In schematic representations of proteins, α helices are depicted as twisted ribbons or rods (Figure 2.27).
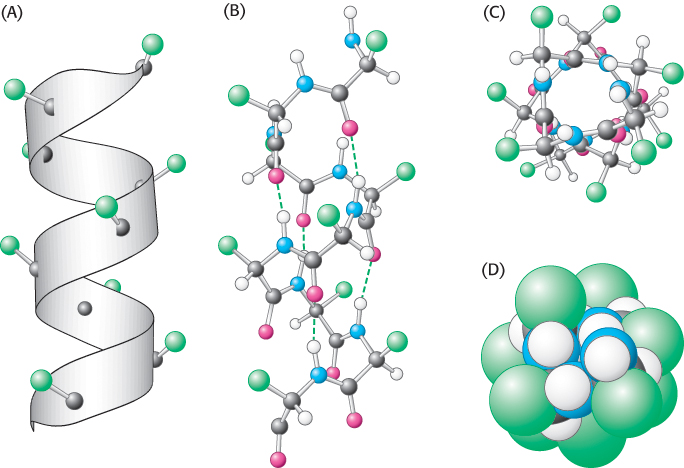
Figure 2.24: Structure of the α helix. (A) A ribbon depiction shows the α-carbon atoms and side chains (green). (B) A side view of a ball-and-stick version depicts the hydrogen bonds (dashed lines) between NH and CO groups. (C) An end view shows the coiled backbone as the inside of the helix and the side chains (green) projecting outward. (D) A space-filling view of part C shows the tightly packed interior core of the helix.
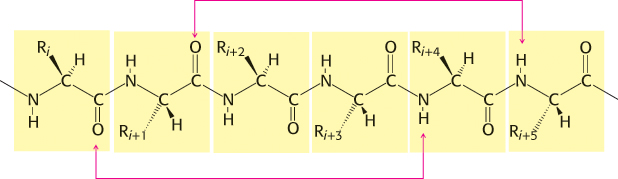
Figure 2.25: Hydrogen-bonding scheme for an α helix. In the α helix, the CO group of residue i forms a hydrogen bond with the NH group of residue i + 4.
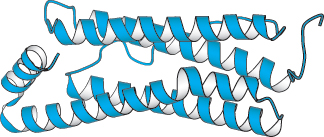
Figure 2.28:  A largely α-helical protein. Ferritin, an iron-storage protein, is built from a bundle of α helices.
A largely α-helical protein. Ferritin, an iron-storage protein, is built from a bundle of α helices.
[Drawn from 1AEW.pdb.]
Not all amino acids can be readily accommodated in an α helix. Branching at the β-carbon atom, as in valine, threonine, and isoleucine, tends to destabilize α helices because of steric clashes. Serine, aspartate, and asparagine also tend to disrupt α helices because their side chains contain hydrogen-bond donors or acceptors in close proximity to the main chain, where they compete for main-chain NH and CO groups. Proline also is a helix breaker because it lacks an NH group and because its ring structure prevents it from assuming the ϕ value to fit into an α helix.
The α-helical content of proteins ranges widely, from none to almost 100%. For example, about 75% of the residues in ferritin, a protein that helps store iron, are in α helices (Figure 2.28). Indeed, about 25% of all soluble proteins are composed of α helices connected by loops and turns of the polypeptide chain. Single α helices are usually less than 45 Å long. Many proteins that span biological membranes also contain α helices.
Beta sheets are stabilized by hydrogen bonding between polypeptide strands
Pauling and Corey proposed another periodic structural motif, which they named the β pleated sheet (β because it was the second structure that they elucidated, the α helix having been the first). The β pleated sheet (or, more simply, the β sheet) differs markedly from the rodlike α helix. It is composed of two or more polypeptide chains called β strands. A β strand is almost fully extended rather than being tightly coiled as in the α helix. A range of extended structures are sterically allowed (Figure 2.29).
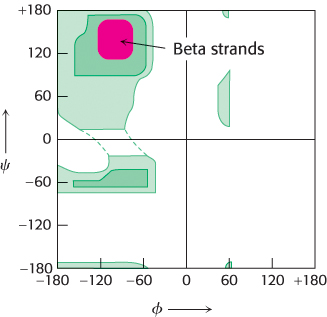
Figure 2.29: Ramachandran plot for β strands. The red area shows the sterically allowed conformations of extended, β-strand-like structures.
The distance between adjacent amino acids along a β strand is approximately 3.5 Å, in contrast with a distance of 1.5 Å along an α helix. The side chains of adjacent amino acids point in opposite directions (Figure 2.30).
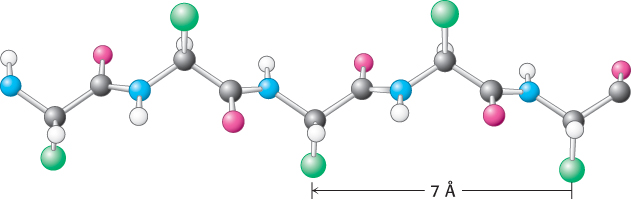
Figure 2.30: Structure of a β strand. The side chains (green) are alternately above and below the plane of the strand.
A β sheet is formed by linking two or more β strands lying next to one another through hydrogen bonds. Adjacent strands in a β sheet can run in opposite directions (antiparallel β sheet) or in the same direction (parallel β sheet). In the antiparallel arrangement, the NH group and the CO group of each amino acid are respectively hydrogen bonded to the CO group and the NH group of a partner on the adjacent chain (Figure 2.31). In the parallel arrangement, the hydrogen-bonding scheme is slightly more complicated. For each amino acid, the NH group is hydrogen bonded to the CO group of one amino acid on the adjacent strand, whereas the CO group is hydrogen bonded to the NH group on the amino acid two residues farther along the chain (Figure 2.32). Many strands, typically 4 or 5 but as many as 10 or more, can come together in β sheets. Such β sheets can be purely antiparallel, purely parallel, or mixed (Figure 2.33).
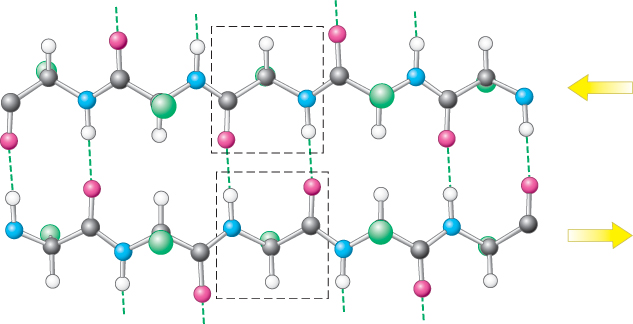
Figure 2.31: An antiparallel β sheet. Adjacent β strands run in opposite directions, as indicated by the arrows. Hydrogen bonds between NH and CO groups connect each amino acid to a single amino acid on an adjacent strand, stabilizing the structure.
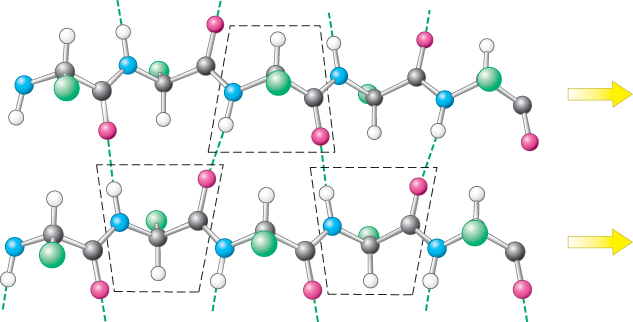
Figure 2.32: A parallel β sheet. Adjacent β strands run in the same direction, as indicated by the arrows. Hydrogen bonds connect each amino acid on one strand with two different amino acids on the adjacent strand.
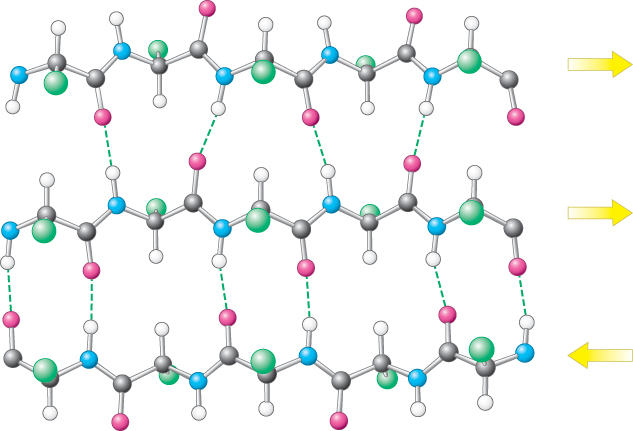
Figure 2.33: Structure of a mixed β sheet. The arrows indicate directionality of each strand.
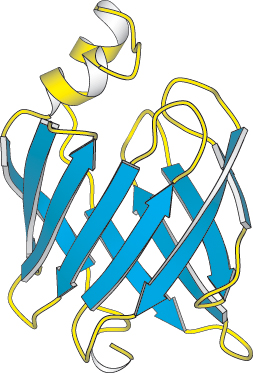
Figure 2.35:  A protein rich in β sheets. The structure of a fatty acid-binding protein.
A protein rich in β sheets. The structure of a fatty acid-binding protein.
[Drawn from 1FTP.pdb.]
In schematic representations, β strands are usually depicted by broad arrows pointing in the direction of the carboxyl-terminal end to indicate the type of β sheet formed—parallel or antiparallel. More structurally diverse than α helices, β sheets can be almost flat but most adopt a somewhat twisted shape (Figure 2.34). The β sheet is an important structural element in many proteins. For example, fatty acid-binding proteins, important for lipid metabolism, are built almost entirely from β sheets (Figure 2.35).
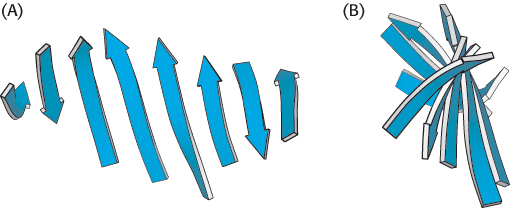
Figure 2.34: A schematic twisted β sheet. (A) A schematic model. (B) The schematic view rotated by 90 degrees to illustrate the twist more clearly.
Polypeptide chains can change direction by making reverse turns and loops
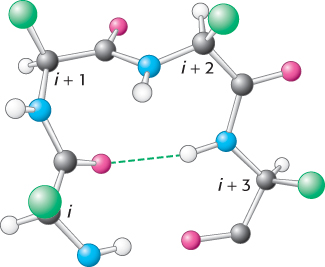
Figure 2.36: Structure of a reverse turn. The CO group of residue i of the polypeptide chain is hydrogen bonded to the NH group of residue i + 3 to stabilize the turn.
Most proteins have compact, globular shapes owing to reversals in the direction of their polypeptide chains. Many of these reversals are accomplished by a common structural element called the reverse turn (also known as the β turn or hairpin turn), illustrated in Figure 2.36. In many reverse turns, the CO group of residue i of a polypeptide is hydrogen bonded to the NH group of residue i + 3. This interaction stabilizes abrupt changes in direction of the polypeptide chain. In other cases, more-elaborate structures are responsible for chain reversals. These structures are called loops or sometimes Ω loops (omega loops) to suggest their overall shape. Unlike α helices and β strands, loops do not have regular, periodic structures. Nonetheless, loop structures are often rigid and well defined (Figure 2.37). Turns and loops invariably lie on the surfaces of proteins and thus often participate in interactions between proteins and other molecules.
Fibrous proteins provide structural support for cells and tissues
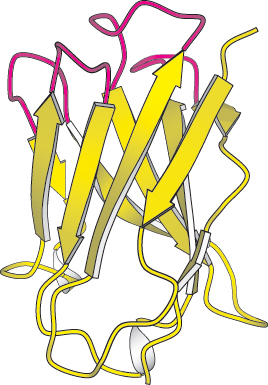
Figure 2.37:  Loops on a protein surface. A part of an antibody molecule has surface loops (shown in red) that mediate interactions with other molecules.
Loops on a protein surface. A part of an antibody molecule has surface loops (shown in red) that mediate interactions with other molecules.
[Drawn from 7FAB.pdb.]
Special types of helices are present in the two proteins α-keratin and collagen. These proteins form long fibers that serve a structural role.
α-Keratin, which is an essential component of wool, hair, and skin, consists of two right-handed α helices intertwined to form a type of left-handed superhelix called an α-helical coiled coil. α-Keratin is a member of a superfamily of proteins referred to as coiled-coil proteins (Figure 2.38). In these proteins, two or more α helices can entwine to form a very stable structure, which can have a length of 1000 Å (100 nm, or 0.1 μm) or more. There are approximately 60 members of this family in humans, including intermediate filaments, proteins that contribute to the cell cytoskeleton (internal scaffolding in a cell), and the muscle proteins myosin and tropomyosin (Section 35.2). Members of this family are characterized by a central region of 300 amino acids that contains imperfect repeats of a sequence of seven amino acids called a heptad repeat.
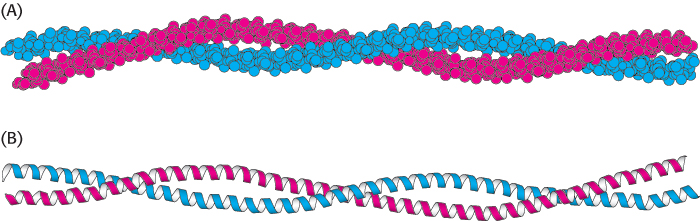
Figure 2.38:  An α-helical coiled coil. (A) Space-filling model. (B) Ribbon diagram. The two helices wind around one another to form a superhelix. Such structures are found in many proteins, including keratin in hair, quills, claws, and horns.
An α-helical coiled coil. (A) Space-filling model. (B) Ribbon diagram. The two helices wind around one another to form a superhelix. Such structures are found in many proteins, including keratin in hair, quills, claws, and horns.
[Drawn from 1C1G.pdb.]
The two helices in α-keratin associate with each other by weak interactions such as van der Waals forces and ionic interactions. The left-handed supercoil alters the two right-handed α helices such that there are 3.5 residues per turn instead of 3.6. Thus, the pattern of side-chain interactions can be repeated every seven residues, forming the heptad repeats. Two helices with such repeats are able to interact with one another if the repeats are complementary (Figure 2.39). For example, the repeating residues may be hydrophobic, allowing van der Waals interactions, or have opposite charge, allowing ionic interactions. In addition, the two helices may be linked by disulfide bonds formed by neighboring cysteine residues. The bonding of the helices accounts for the physical properties of wool, an example of an α-keratin. Wool is extensible and can be stretched to nearly twice its length because the α helices stretch, breaking the weak interactions between neighboring helices. However, the covalent disulfide bonds resist breakage and return the fiber to its original state once the stretching force is released. The number of disulfide bond cross-links further defines the fiber’s properties. Hair and wool, having fewer cross-links, are flexible. Horns, claws, and hooves, having more cross-links, are much harder.
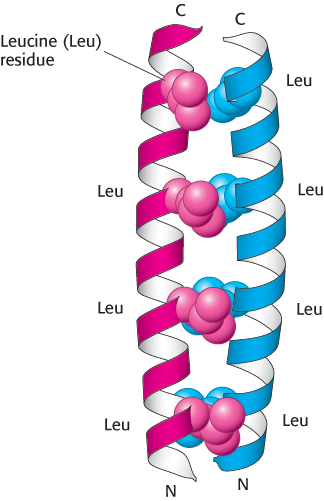
Figure 2.39:  Heptad repeats in a coiled-coil protein. Every seventh residue in each helix is leucine. The two helices are held together by van der Waals interactions primarily between the leucine residues.
Heptad repeats in a coiled-coil protein. Every seventh residue in each helix is leucine. The two helices are held together by van der Waals interactions primarily between the leucine residues.
[Drawn from 2ZTA.pdb.]
A different type of helix is present in collagen, the most abundant protein of mammals. Collagen is the main fibrous component of skin, bone, tendon, cartilage, and teeth. This extracellular protein is a rod-shaped molecule, about 3000 Å long and only 15 Å in diameter. It contains three helical polypeptide chains, each nearly 1000 residues long. Glycine appears at every third residue in the amino acid sequence, and the sequence glycine-proline-hydroxyproline recurs frequently (Figure 2.40). Hydroxyproline is a derivative of proline that has a hydroxyl group in place of one of the hydrogen atoms on the pyrrolidine ring.

Figure 2.40: Amino acid sequence of a part of a collagen chain. Every third residue is a glycine. Proline and hydroxyproline (Hyp) also are abundant.
The collagen helix has properties different from those of the α helix. Hydrogen bonds within a strand are absent. Instead, the helix is stabilized by steric repulsion of the pyrrolidine rings of the proline and hydroxyproline residues (Figure 2.41). The pyrrolidine rings keep out of each other’s way when the polypeptide chain assumes its helical form, which has about three residues per turn. Three strands wind around one another to form a superhelical cable that is stabilized by hydrogen bonds between strands. The hydrogen bonds form between the peptide NH groups of glycine residues and the CO groups of residues on the other chains. The hydroxyl groups of hydroxyproline residues also participate in hydrogen bonding.
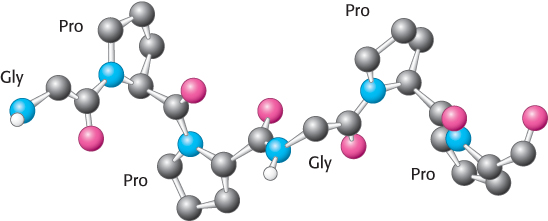
Figure 2.41: Conformation of a single strand of a collagen triple helix.
The inside of the triple-stranded helical cable is very crowded and accounts for the requirement that glycine be present at every third position on each strand (Figure 2.42A). The only residue that can fit in an interior position is glycine. The amino acid residue on either side of glycine is located on the outside of the cable, where there is room for the bulky rings of proline and hydroxyproline residues (Figure 2.42B).
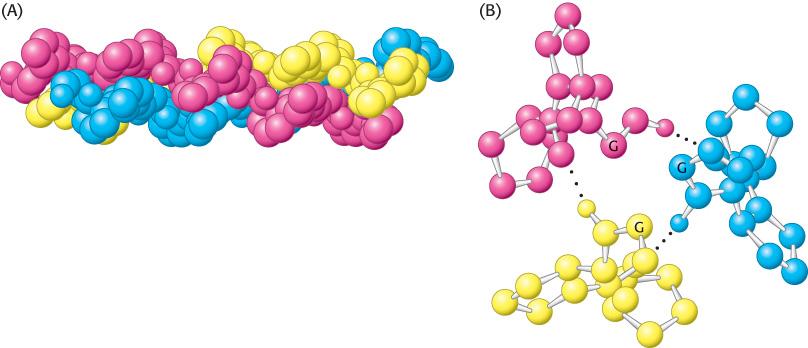
Figure 2.42: Structure of the protein collagen. (A) Space-filling model of collagen. Each strand is shown in a different color. (B) Cross section of a model of collagen. Each strand is hydrogen bonded to the other two strands. The α-carbon atom of a glycine residue is identified by the letter G. Every third residue must be glycine because there is no space in the center of the helix. Notice that the pyrrolidine rings of the proline residues are on the outside.
 The importance of the positioning of glycine inside the triple helix is illustrated in the disorder osteogenesis imperfecta, also known as brittle bone disease. In this condition, which can vary from mild to very severe, other amino acids replace the internal glycine residue. This replacement leads to a delayed and improper folding of collagen. The most serious symptom is severe bone fragility. Defective collagen in the eyes causes the whites of the eyes to have a blue tint (blue sclera).
The importance of the positioning of glycine inside the triple helix is illustrated in the disorder osteogenesis imperfecta, also known as brittle bone disease. In this condition, which can vary from mild to very severe, other amino acids replace the internal glycine residue. This replacement leads to a delayed and improper folding of collagen. The most serious symptom is severe bone fragility. Defective collagen in the eyes causes the whites of the eyes to have a blue tint (blue sclera).
 H and C
H and C O groups of amino acids that are near one another in the linear sequence. Such folded segments are called secondary structure.
O groups of amino acids that are near one another in the linear sequence. Such folded segments are called secondary structure.




 A largely α-helical protein. Ferritin, an iron-
A largely α-helical protein. Ferritin, an iron-





 A protein rich in β sheets. The structure of a fatty acid-
A protein rich in β sheets. The structure of a fatty acid-


 Loops on a protein surface. A part of an antibody molecule has surface loops (shown in red) that mediate interactions with other molecules.
Loops on a protein surface. A part of an antibody molecule has surface loops (shown in red) that mediate interactions with other molecules.

 An α-helical coiled coil. (A) Space-
An α-helical coiled coil. (A) Space-
 Heptad repeats in a coiled-
Heptad repeats in a coiled-


 The importance of the positioning of glycine inside the triple helix is illustrated in the disorder osteogenesis imperfecta, also known as brittle bone disease. In this condition, which can vary from mild to very severe, other amino acids replace the internal glycine residue. This replacement leads to a delayed and improper folding of collagen. The most serious symptom is severe bone fragility. Defective collagen in the eyes causes the whites of the eyes to have a blue tint (blue sclera).
The importance of the positioning of glycine inside the triple helix is illustrated in the disorder osteogenesis imperfecta, also known as brittle bone disease. In this condition, which can vary from mild to very severe, other amino acids replace the internal glycine residue. This replacement leads to a delayed and improper folding of collagen. The most serious symptom is severe bone fragility. Defective collagen in the eyes causes the whites of the eyes to have a blue tint (blue sclera).