7.3 Hydrogen Ions and Carbon Dioxide Promote the Release of Oxygen: The Bohr Effect
We have seen how cooperative release of oxygen from hemoglobin helps deliver oxygen to where it is most needed: tissues exhibiting low oxygen partial pressures. This ability is enhanced by the facility of hemoglobin to respond to other cues in its physiological environment that signal the need for oxygen. Rapidly metabolizing tissues, such as contracting muscle, generate large amounts of hydrogen ions and carbon dioxide (Chapter 16). To release oxygen where the need is greatest, hemoglobin has evolved to respond to higher levels of these substances. Like 2,3-
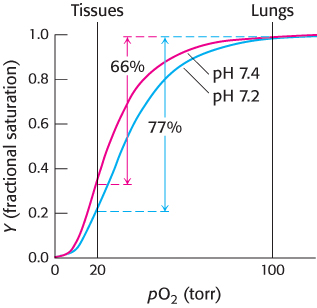
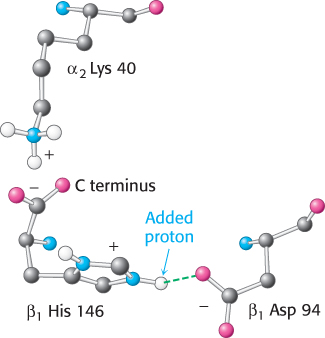
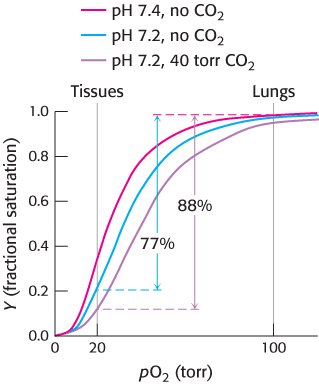
The oxygen affinity of hemoglobin decreases as pH decreases from a value of 7.4 (Figure 7.19). Consequently, as hemoglobin moves into a region of lower pH, its tendency to release oxygen increases. For example, transport from the lungs, with pH 7.4 and an oxygen partial pressure of 100 torr, to active muscle, with a pH of 7.2 and an oxygen partial pressure of 20 torr, results in a release of oxygen amounting to 77% of total carrying capacity. Only 66% of the oxygen would be released in the absence of any change in pH. Structural and chemical studies have revealed much about the chemical basis of the Bohr effect. Several chemical groups within the hemoglobin tetramer are important for sensing changes in pH; all of these have pKa values near pH 7. Consider histidine β146, the residue at the C terminus of the β chain. In deoxyhemoglobin, the terminal carboxylate group of β146 forms a ionic bond, also called a salt bridge, with a lysine residue in the α subunit of the other αβ dimer. This interaction locks the side chain of histidine β146 in a position from which it can participate in a salt bridge with negatively charged aspartate β94 in the same chain, provided that the imidazole group of the histidine residue is protonated (Figure 7.20).
203
In addition to His β146, the α-amino groups at the amino termini of the α chain and the side chain of histidine α122 also participate in salt bridges in the T state. The formation of these salt bridges stabilizes the T state, leading to a greater tendency for oxygen to be released. For example, at high pH, the side chain of histidine β146 is not protonated and the salt bridge does not form. As the pH drops, however, the side chain of histidine β146 becomes protonated, the salt bridge with aspartate β94 forms, and the T state is stabilized.
Carbon dioxide, a neutral species, passes through the red-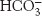 , and H+, resulting in a drop in pH that stabilizes the T state by the mechanism discussed previously.
, and H+, resulting in a drop in pH that stabilizes the T state by the mechanism discussed previously.
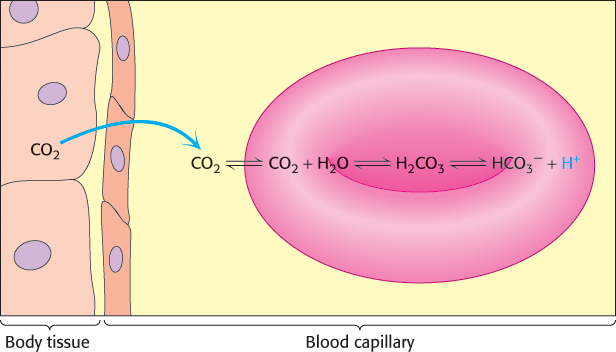
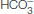 and H+, resulting in a drop in pH inside the red cell.
and H+, resulting in a drop in pH inside the red cell.
204
In the second mechanism, a direct chemical interaction between carbon dioxide and hemoglobin stimulates oxygen release. The effect of carbon dioxide on oxygen affinity can be seen by comparing oxygen-
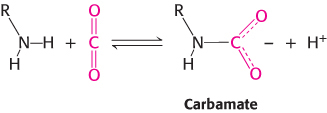
The amino termini lie at the interface between the αβ dimers, and these negatively charged carbamate groups participate in salt-
Carbamate formation also provides a mechanism for carbon dioxide transport from tissues to the lungs, but it accounts for only about 14% of the total carbon dioxide transport. Most carbon dioxide released from red blood cells is transported to the lungs in the form of 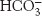 produced from the hydration of carbon dioxide inside the cell (Figure 7.23). Much of the
produced from the hydration of carbon dioxide inside the cell (Figure 7.23). Much of the 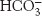 that is formed leaves the cell through a specific membrane-
that is formed leaves the cell through a specific membrane-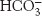 from one side of the membrane for Cl− from the other side. Thus, the serum concentration of
from one side of the membrane for Cl− from the other side. Thus, the serum concentration of 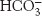 increases. By this means, a large concentration of carbon dioxide is transported from tissues to the lungs in the form of
increases. By this means, a large concentration of carbon dioxide is transported from tissues to the lungs in the form of 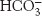 . In the lungs, this process is reversed:
. In the lungs, this process is reversed: 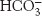 is converted back into carbon dioxide and exhaled. Thus, carbon dioxide generated by active tissues contributes to a decrease in red-
is converted back into carbon dioxide and exhaled. Thus, carbon dioxide generated by active tissues contributes to a decrease in red-
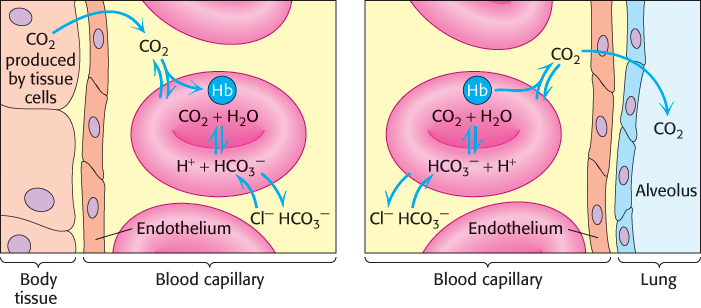
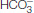 produced in red blood cells and then released into the blood plasma. A lesser amount is transported by hemoglobin in the form of an attached carbamate.
produced in red blood cells and then released into the blood plasma. A lesser amount is transported by hemoglobin in the form of an attached carbamate.