8.6 Enzymes Can Be Studied One Molecule at a Time
Most experiments that are performed to determine an enzyme characteristic require an enzyme preparation in a buffered solution. Even a few microliters of such a solution will contain millions of enzyme molecules. Much that we have learned about enzymes thus far has come from such experiments, called ensemble studies. A basic assumption of ensemble studies is that all of the enzyme molecules are the same or very similar. When we determine an enzymatic property such as the value of KM in ensemble studies, that value is of necessity an average value of all of the enzyme molecules present. However, as discussed in Chapter 2, we now know that molecular heterogeneity, the ability of a molecule, over time, to assume several different structures that differ slightly in stability, is an inherent property of all large biomolecules. How can we tell if this molecular heterogeneity affects enzyme activity?
By way of example, consider a hypothetical situation. A Martian visits Earth to learn about higher education. The spacecraft hovers high above a university, and our Martian meticulously records how the student population moves about campus. Much information can be gathered from such studies: where students are likely to be at certain times on certain days, which buildings are used when and by how many. Now, suppose our visitor developed a high-
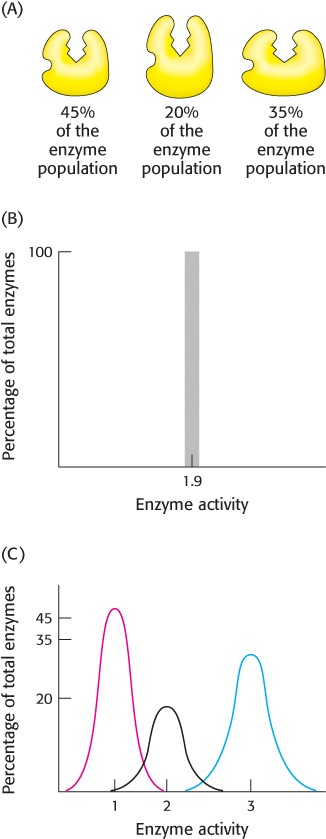
Let us leave our Martian to his observations, and consider a more biochemical situation. Figure 8.34A shows an enzyme that displays molecular heterogeneity, with three active forms that catalyze the same reaction but at different rates. These forms have slightly different stabilities, but thermal noise is sufficient to interconvert the forms. Each form is present as a fraction of the total enzyme population as indicated. If we were to perform an experiment to determine enzyme activity under a particular set of conditions with the use of ensemble methods, we would get a single value, which would represent the average of the heterogeneous assembly (Figure 8.34B). However, were we to perform a sufficient number of single-
The development of powerful techniques—
243