Chapter 11
1. Carbohydrates were originally regarded as hydrates of carbon because the empirical formula of many of them is (CH2O)n.
2. Three amino acids can be linked by peptide bonds in only six different ways. However, three different monosaccharides can be linked in a plethora of ways. The monosaccharides can be linked in a linear or branched manner, with α or β linkages, with bonds between C-
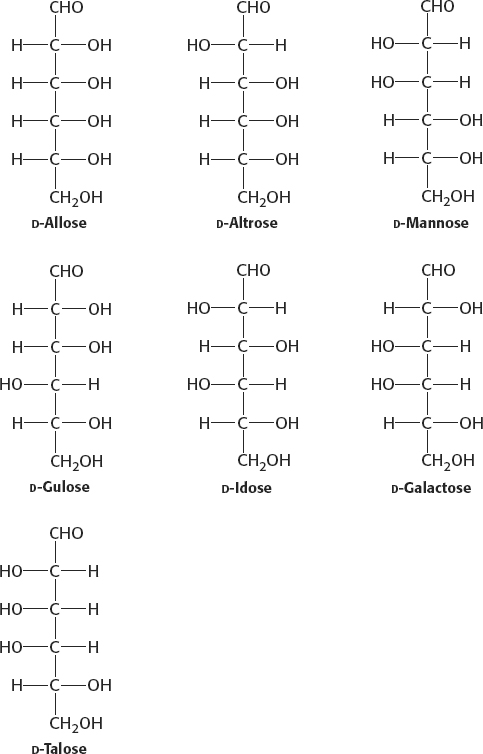
3. (a) 10; (b) 6; (c) 8; (d) 9; (e) 2; (f) 4; (g) 1; (h) 5; (i) 7; (j) 3.
4. (a) aldose-
5. Erythrose: tetrose aldose; ribose: pentose aldose; glyceraldehyde: triose aldose; dihydroxyacetone: triose ketose; erythrulose: tetrose ketose; ribulose: pentose ketose; fructose: hexose ketose.
6.
7.
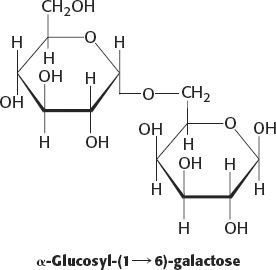
8. The proportion of the α anomer is 0.36, and that of the β anomer is 0.64.
9. Glucose is reactive because of the presence of an aldehyde group in its open-
A14
10. A pyranoside reacts with two molecules of periodate; formate is one of the products. A furanoside reacts with only one molecule of periodate; formate is not formed.
11. (a) β-d-Mannose; (b) β-d-galactose; (c) β-d-fructose; (d) β-d-glucosamine.
12. The trisaccharide itself should be a competitive inhibitor of cell adhesion if the trisaccharide unit of the glycoprotein is critical for the interaction.
13. Reducing ends would form 1,2,3,6-
14. (a) Not a reducing sugar; no open-
15.
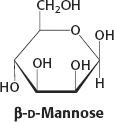
The hemiketal linkage of the α anomer is broken to form the open form. Rotation about the C-
16. Heating converts the very sweet pyranose form into the more-
17. (a) Each glycogen molecule has one reducing end, whereas the number of nonreducing ends is determined by the number of branches, or α-1,6 linkages. (b) Because the number of nonreducing ends greatly exceeds the number of reducing ends in a collection of glycogen molecules, all of the degradation and synthesis of glycogen takes place at the nonreducing ends, thus maximizing the rate of degradation and synthesis.
18. No, sucrose is not a reducing sugar. The anomeric carbon atom acts as the reducing agent in both glucose and fructose but, in sucrose, the anomeric carbon atoms of fructose and glucose are joined by a covalent bond and are thus not available to react.
19. Glycogen is a polymer of glucose linked by α-1,4-
20. Cellulose is a linear polymer of glucose joined by β-1,4 linkages. Glycogen is a branched polymer with the main chain being formed by α-1,4-
21. Simple glycoproteins are often secreted proteins and thus play a variety of roles. For example, the hormone EPO is a glycoprotein. Usually, the protein component constitutes the bulk of the glycoprotein by mass. In contrast, proteoglycans and mucoproteins are predominantly carbohydrates. Proteoglycans have glycosaminoglycans attached, and play structural roles as in cartilage and the extracellular matrix. Mucoproteins often serve as lubricants and have multiple carbohydrates attached through an N-acetylgalactosamine moiety.
22. The attachment of the carbohydrate allows the EPO to stay in circulation longer and thus to function for longer periods of time than would a carbohydrate-
23. The glycosaminoglycan, because it is heavily charged, binds many water molecules. When cartilage is stressed, such as when your heel hits the ground, the water is released, thus cushioning the impact. When you lift your heel, the water rebinds.
24. The lectin that binds the mannose 6-
25. Asparagine, serine, and threonine.
26. Different molecular forms of a glycoprotein that differ in the amount of carbohydrate attached or the location of attachment or both.
27. The total collection of carbohydrates synthesized by a cell at particular times and under particular environmental conditions.
28. The genome comprises all of the genes present in an organism. The proteome includes all of the possible protein products and modified proteins that a cell expresses under any particular set of circumstances. The glycome consists of all of the carbohydrates synthesized by the cell under any particular set of circumstances. Because the genome is static, but any given protein can be variously expressed and modified, the proteome is more complex than the genome. The glycome, which includes not only glycoforms of proteins, but also many possible carbohydrate structures, must be even more complex.
29. An asparagine residue can be glycosylated if the residue is part of an Asn-
30. It suggests that carbohydrates are on the cell surfaces of all organisms for the purpose of recognition by other cells, organisms, or the environment.
31. A glycoprotein is a protein that is decorated with carbohydrates. A lectin is a protein that specifically recognizes carbohydrates. A lectin can also be a glycoprotein.
32. Each site either is or is not glycosylated, and so there are 26 = 64 possible proteins.
33. The 20 amino acid components of proteins and the 4 nucleotide components of nucleic acids are linked by the same type of bond—
34. As discussed in Chapter 9, many enzymes display stereochemical specificity. Clearly, the enzymes of sucrose synthesis are able to distinguish between the isomers of the substrates and link only the correct pair.
35. If the carbohydrate specificity of the lectin is known, an affinity column with the appropriate carbohydrate attached could be prepared. The protein preparation containing the lectin of interest could be passed over the column. The use of this method was indeed how the glucose-
36. (a) Aggrecan is heavily decorated with glycosaminoglycans. If glycosaminoglycans are released into the media, aggrecan must be undergoing degradation.
(b) Another enzyme might be present that cleaves glycosaminoglycans from aggrecan without degrading aggrecan. Other experiments not shown established that glycosaminoglycan release is an accurate measure of aggrecan destruction.
(c) The control provides a baseline of “background” degradation inherent in the assay.
(d) Aggrecan degradation is greatly enhanced.
A15
(e) Aggrecan degradation is reduced to the background system.
(f) It is an in vitro system in which not all the factors contributing to cartilage stabilization in vivo are present.