Chapter 13
1. In simple diffusion, the substance in question can diffuse down its concentration gradient through the membrane. In facilitated diffusion, the substance is not lipophilic and cannot directly diffuse through the membrane. A channel or carrier is required to facilitate movement down the gradient.
A16
2. The two forms are (1) ATP hydrolysis and (2) the movement of one molecule down its concentration gradient coupled with the movement of another molecule up its concentration gradient.
3. The three types of carriers are symporters, antiporters, and uniporters. Symporters and antiporters can mediate secondary active transport.
4. The free-
5. For chloride, z = −1; for calcium z = +2. At the concentrations given, the equilibrium potential for chloride is −97 mV and the equlibrium potential for calcium is +122 mV.
6. The concentration of glucose inside the cell is 66 times as great as that outside the cell [(c2/c1) = 66] when the free-
7. By analogy with the Ca2+ ATPase, with three Na+ ions binding from inside the cell to the E1 conformation and with two K+ ions binding from outside the cell to the E2 conformation, a plausible mechanism is as follows:
(i) A catalytic cycle could begin with the enzyme in its unphosphorylated state (E1) with three sodium ions bound.
(ii) The E1 conformation binds ATP. A conformational change traps sodium ions inside the enzyme.
(iii) The phosphoryl group is transferred from ATP to an aspartyl residue.
(iv) On ADP release, the enzyme changes its overall conformation, including the membrane domain. This new conformation (E2) releases the sodium ions to the side of the membrane opposite that at which they entered and binds two potassium ions from the side where sodium ions are released.
(v) The phosphorylaspartate residue is hydrolyzed to release inorganic phosphate. With the release of phosphate, the interactions stabilizing E2 are lost, and the enzyme everts to the E1 conformation. Potassium ions are released to the cytoplasmic side of the membrane. The binding of three sodium ions from the cytoplasmic side of the membrane completes the cycle.
8. Establish a lactose gradient across vesicle membranes that contain properly oriented lactose permease. Initially, the pH should be the same on both sides of the membrane and the lactose concentration should be higher on the “exit” side of lactose permease. As the lactose flows “in reverse” through the permease, down its concentration gradient, it can be tested whether or not a pH gradient becomes established as the lactose gradient is dissipated.
9. Ligand-
10. An ion channel must transport ions in either direction at the same rate. The net flow of ions is determined only by the composition of the solutions on either side of the membrane.
11. Uniporters act as enzymes do; their transport cycles include large conformational changes, and only a few molecules interact with the protein per transport cycle. In contrast, channels, after having opened, provide a pore in the membrane through which many ions may pass. As such, channels mediate transport at a much higher rate than do uniporters.
12. FCCP effectively creates a pore in the bacterial membrane through which protons can pass rapidly. Protons that are pumped out of the bacteria will pass through this pore preferentially (the “path of least resistance”), rather than participate in H+/lactose symport.
13. Cardiac muscle must contract in a highly coordinated manner in order to pump blood effectively. Gap junctions mediate the orderly cell-
14. The positively charged guanidinium group resembles Na+ and binds to negatively charged carboxylate groups in the mouth of the channel.
15. SERCA, a P-
16. The blockage of ion channels inhibits action potentials, leading to loss of nervous function. Like tetrodotoxin, these toxin molecules are useful for isolating and specifically inhibiting particular ion channels.
17. After repolarization, the ball domains of the ion channels engage the channel pore, rendering them inactive for a short period of time. During this time, the channels cannot be reopened until the ball domains disengage and the channel returns to the “closed” state.
18. Because sodium ions are charged and because sodium channels carry only sodium ions (but not anions), the accumulation of excess positive charge on one side of the membrane dominates the chemical gradients.
19. A mutation that impairs the ability of the sodium channel to inactivate would prolong the duration of the depolarizing sodium current, thus lengthening the cardiac action potential.
20. No. Channels will likely open or close in response to an external stimulus, but the unit conductance of the open channel will be influenced very little.
21. The ratio of closed to open forms of the channel is 105, 5000, 250, 12.5, and 0.625 when zero, one, two, three, and four ligands, respectively, are bound. Hence, the fraction of open channels is 1.0 × 10−5, 2.0 × 10−4, 4.0 × 10−3, 7.4 × 10−2, and 0.62.
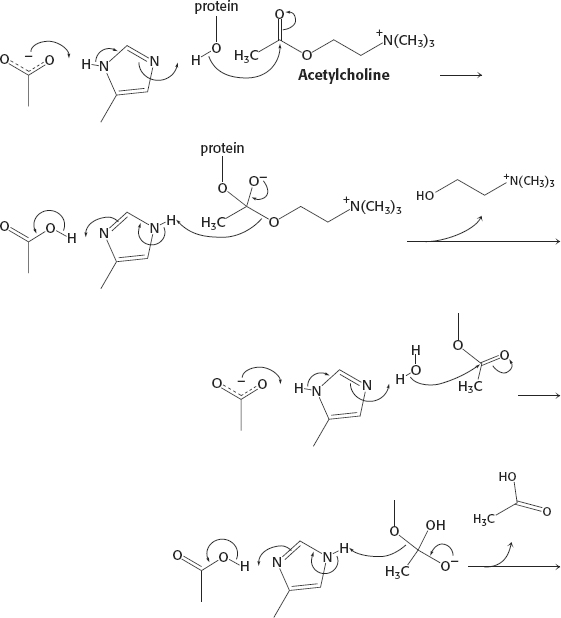
22. These organic phosphates inhibit acetylcholinesterase by reacting with the active-
23. (a) The binding of the first acetylcholine molecule increases the open-
24. Batrachotoxin blocks the transition from the open to the closed state.
25. (a) Chloride ions flow into the cell. (b) Chloride flux is inhibitory because it hyperpolarizes the membrane. (c) The channel consists of five subunits.
26. After the addition of ATP and calcium, SERCA will pump Ca2+ ions into the vesicle. However, the accumulation of Ca2+ ions inside the vesicle will rapidly lead to the formation of an electrical gradient that cannot be overcome by ATP hydrolysis. The addition of calcimycin will allow the pumped Ca2+ ions to flow back out of the vesicle, dissipating the charge buildup, and enabling the pump to operate continuously.
27. The catalytic prowess of acetylcholinesterase ensures that the duration of the nerve stimulus will be short.
A17
28. See reaction below.
29. (a) Only ASIC1a is inhibited by the toxin. (b) Yes; when the toxin was removed, the activity of the acid-
30. This mutation is one of a class of mutations that result in slow-
31. The mutation reduces the affinity of acetylcholine for the receptor. The recordings would show the channel opening only infrequently.
32. Glucose displays a transport curve that suggests the participation of a carrier because the initial rate is high but then levels off at higher concentrations, consistent with saturation of the carrier, which is reminiscent of Michaelis–